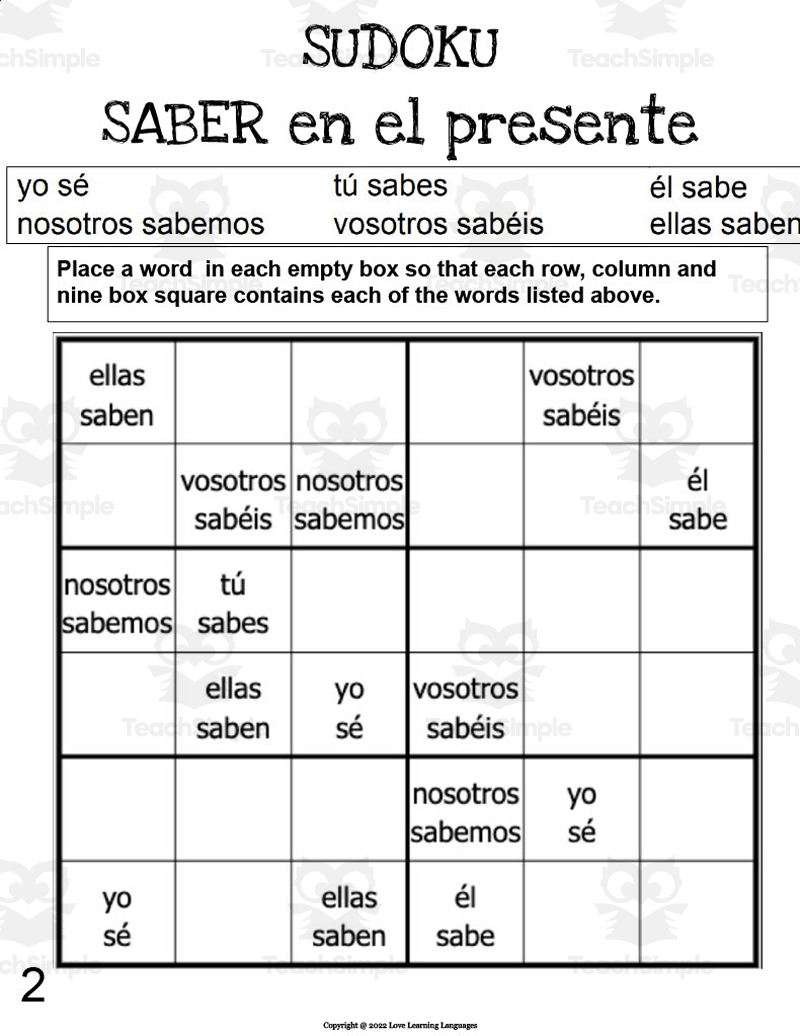
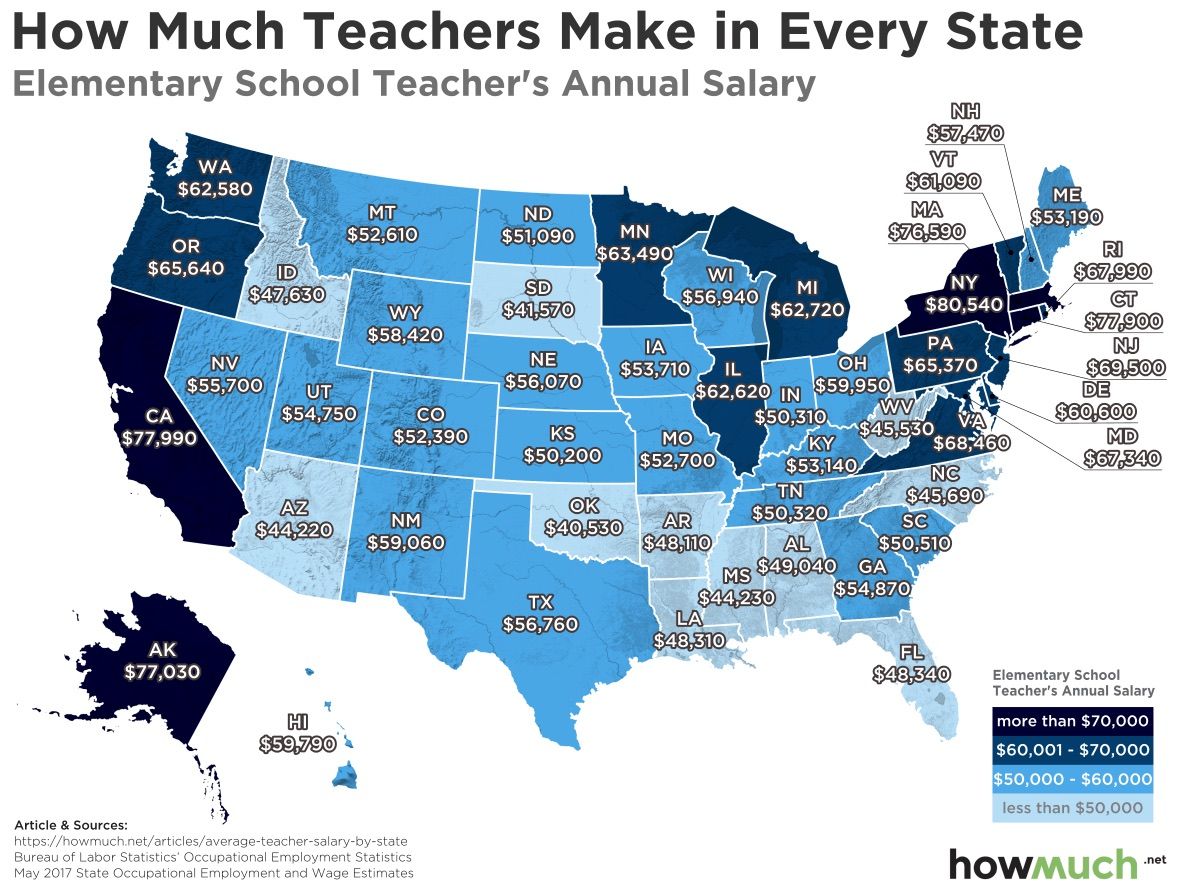
Understanding Ammonia's Conjugate Base: A Quick Guide

Ammonia, a common household cleaner and an essential compound in various industries, plays a significant role in chemistry due to its ability to act as a base. Understanding its conjugate base is crucial for anyone delving into acid-base chemistry, whether for academic purposes or practical applications. This guide will walk you through the basics of ammonia’s conjugate base, its properties, and its importance in chemical reactions, all while optimizing for SEO to ensure you find the information you need quickly and efficiently.
What is Ammonia’s Conjugate Base?

Ammonia (NH₃) is a weak base that readily accepts a proton (H⁺) in aqueous solutions. When it does so, it forms its conjugate acid, the ammonium ion (NH₄⁺). Conversely, the conjugate base of ammonia is the species that remains after ammonia donates a proton. However, ammonia itself does not typically donate protons; instead, it accepts them. Therefore, the conjugate base of ammonia is not directly formed in this manner.
To clarify, the conjugate base of ammonia would theoretically be NH₂⁻, but this species is highly unstable and rarely observed in practice. Instead, the focus is often on ammonia’s role as a base and its conjugate acid, NH₄⁺.
💡 Note: The conjugate base of ammonia (NH₂⁻) is unstable and not commonly encountered in chemical reactions.
Properties of Ammonia and Its Conjugate Acid

Ammonia is a colorless gas with a pungent odor, highly soluble in water. When dissolved, it forms ammonium hydroxide (NH₄OH), which dissociates into NH₄⁺ and OH⁻ ions, making the solution basic. The ammonium ion (NH₄⁺), ammonia’s conjugate acid, is a key player in buffer systems and plays a role in biological processes.
- Ammonia (NH₃): Weak base, accepts protons.
- Ammonium ion (NH₄⁺): Conjugate acid of ammonia, forms in aqueous solutions.
| Compound | Role | Stability |
|---|---|---|
| NH₃ | Weak Base | Stable |
| NH₄⁺ | Conjugate Acid | Stable |
| NH₂⁻ | Theoretical Conjugate Base | Unstable |

Applications of Ammonia and Its Conjugate Acid

Ammonia and its conjugate acid, NH₄⁺, have numerous applications across industries:
- Household Cleaning: Ammonia is a common ingredient in glass and surface cleaners.
- Agriculture: Used in fertilizers to provide nitrogen to plants.
- Chemical Manufacturing: Serves as a precursor for various chemicals, including pharmaceuticals.
- Biological Processes: NH₄⁺ is involved in nitrogen metabolism in living organisms.
Key Takeaways

- Ammonia (NH₃) acts as a weak base, accepting protons to form NH₄⁺.
- The conjugate base of ammonia, NH₂⁻, is unstable and rarely observed.
- NH₄⁺, the conjugate acid, is stable and widely used in chemistry and industry.
📌 Note: For practical purposes, focus on ammonia’s role as a base and its conjugate acid, NH₄⁺, rather than its theoretical conjugate base.
Checklist for Understanding Ammonia’s Conjugate Base

- Identify Ammonia’s Role: Recognize NH₃ as a weak base.
- Understand Conjugate Acid: Know NH₄⁺ as the conjugate acid of ammonia.
- Applications: Familiarize yourself with ammonia’s uses in cleaning, agriculture, and manufacturing.
- Stability: Note the instability of NH₂⁻ and its negligible role in reactions.
In summary, while ammonia’s conjugate base (NH₂⁻) is theoretically interesting, its instability makes it less relevant in practical chemistry. Instead, focus on ammonia’s role as a base and the properties of its conjugate acid, NH₄⁺, which are essential in various applications.
What is the conjugate base of ammonia?
+Theoretically, the conjugate base of ammonia is NH₂⁻, but it is highly unstable and rarely observed.
What is the conjugate acid of ammonia?
+The conjugate acid of ammonia is the ammonium ion (NH₄⁺), formed when ammonia accepts a proton.
Why is NH₂⁻ unstable?
+NH₂⁻ is unstable due to the high electronegativity of nitrogen and the lack of a positive charge to stabilize the negative charge.
What are the main uses of ammonia?
+Ammonia is used in cleaning products, fertilizers, chemical manufacturing, and biological processes.
ammonia properties,conjugate acid,weak base,chemical applications,ammonium ion,NH₄⁺,NH₂⁻ stability