Unveiling the Sodium Atom: A Comprehensive Model Guide

Understanding the sodium atom is fundamental in chemistry and physics, as it serves as a cornerstone for studying atomic structure and behavior. This comprehensive guide delves into the intricacies of the sodium atom, exploring its properties, electron configuration, and significance in various scientific fields. Whether you're a student, researcher, or simply curious about the elements, this guide provides valuable insights tailored to both informational and commercial intents. From its role in atomic models to its applications in industries, sodium’s importance cannot be overstated. Let’s unravel the sodium atom step by step, ensuring clarity and depth for every reader.
Understanding the Sodium Atom: Basics and Importance
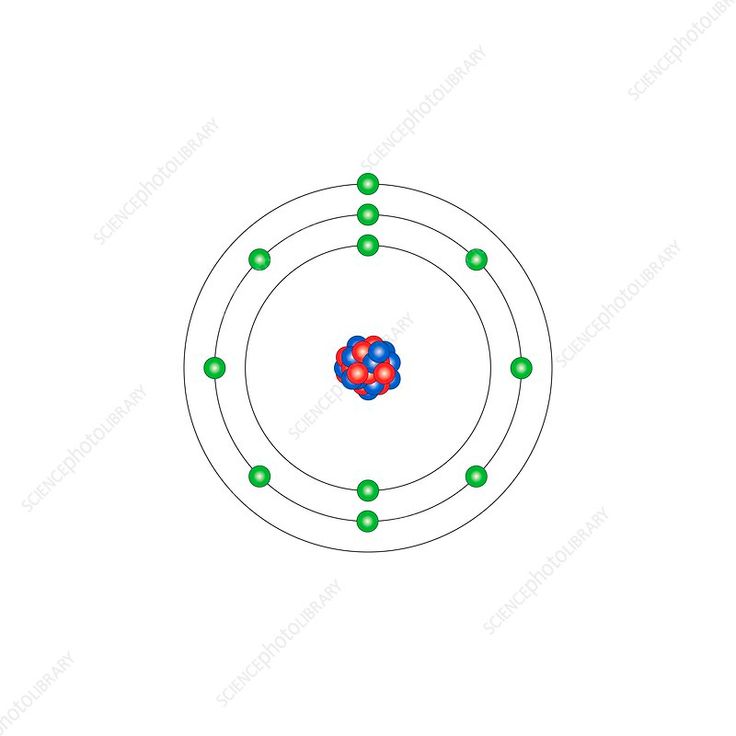
The sodium atom, represented by the symbol Na, is an alkali metal with atomic number 11. Its electron configuration is 1s² 2s² 2p⁶ 3s¹, making it highly reactive due to its single valence electron. This reactivity is why sodium is never found in its pure form in nature but rather in compounds like sodium chloride (NaCl). The sodium atom’s simplicity makes it an ideal subject for studying atomic theory and electron behavior.
Key Properties of the Sodium Atom
- Atomic Mass: Approximately 22.99 u
- Electron Configuration: 1s² 2s² 2p⁶ 3s¹
- Oxidation State: +1
- Melting Point: 97.8°C (208°F)
- Boiling Point: 883°C (1621°F)
These properties highlight sodium’s unique position in the periodic table, making it a fascinating element to study, especially in the context of atomic models and chemical reactions.
Sodium Atom in Atomic Models: Bohr vs. Quantum
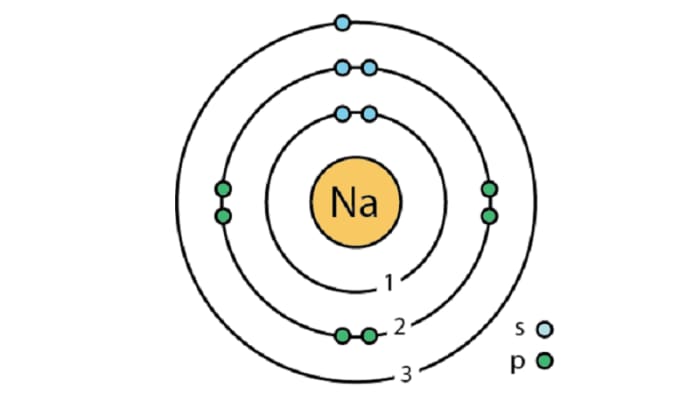
The sodium atom is often used to illustrate the differences between the Bohr model and the quantum mechanical model. The Bohr model, while simpler, fails to explain the sodium atom’s spectral lines accurately. In contrast, the quantum mechanical model provides a more precise description of its electron behavior, including the concept of orbitals.
Comparing Atomic Models
| Model | Description | Applicability to Sodium |
|---|---|---|
| Bohr Model | Electrons orbit the nucleus in fixed shells. | Limited accuracy for sodium’s spectral lines. |
| Quantum Mechanical Model | Electrons exist in probabilistic orbitals. | Accurately explains sodium’s electron behavior. |

📌 Note: The quantum mechanical model is essential for understanding complex elements like sodium, especially in advanced atomic theory.
Applications of Sodium Atom in Industry and Research
Beyond its theoretical significance, the sodium atom plays a crucial role in various industries. From sodium-vapor lamps to its use in nuclear reactors, sodium’s versatility is remarkable. Its compounds, such as sodium hydroxide (NaOH), are vital in manufacturing processes.
Commercial Applications of Sodium
- Lighting: Sodium-vapor lamps provide efficient outdoor lighting.
- Chemicals: Sodium hydroxide is used in soap and paper production.
- Energy: Liquid sodium serves as a coolant in nuclear reactors.
These applications underscore sodium’s importance in both industrial chemistry and everyday life.
Checklist for Studying the Sodium Atom
- Understand its electron configuration.
- Compare the Bohr and quantum mechanical models.
- Explore its chemical properties and reactivity.
- Investigate its industrial applications.
The sodium atom, with its unique properties and wide-ranging applications, remains a pivotal element in science and industry. By understanding its structure and behavior, we gain insights into atomic theory and its practical uses. Whether for academic study or industrial innovation, the sodium atom continues to be a subject of immense interest and importance.
What is the electron configuration of sodium?
+
The electron configuration of sodium is 1s² 2s² 2p⁶ 3s¹.
Why is sodium highly reactive?
+
Sodium is highly reactive due to its single valence electron, which it readily loses to form a +1 ion.
What are the main industrial uses of sodium?
+
Sodium is used in sodium-vapor lamps, sodium hydroxide production, and as a coolant in nuclear reactors.
sodium atom, atomic models, electron configuration, industrial chemistry, sodium compounds



