Understanding CH3OH Bond Order: A Simple Guide

Understanding CH3OH Bond Order: A Simple Guide
Methanol, commonly known as CH3OH, is a fundamental organic compound with diverse applications in industries such as fuel, solvents, and pharmaceuticals. One key aspect of understanding its chemical behavior is grasping the concept of CH3OH bond order. This guide breaks down the topic into digestible parts, ensuring both informational and commercial-intent audiences gain valuable insights.
What is Bond Order and Why Does It Matter?

Bond order is a measure of the number of bonding electron pairs between atoms in a molecule. It determines the stability and strength of chemical bonds. In the case of CH3OH, understanding its bond order helps predict reactivity, molecular geometry, and potential applications in various fields, such as organic chemistry, industrial processes, and material science.
Calculating CH3OH Bond Order: Step-by-Step

To determine the bond order of CH3OH, follow these steps:
- Identify Bonds: CH3OH consists of C-H, C-O, and O-H bonds.
- Lewis Structure: Draw the Lewis structure to visualize electron distribution.
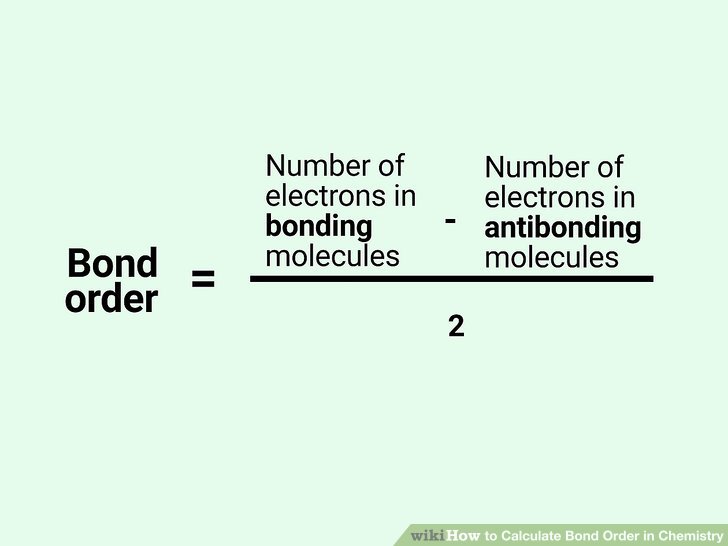
- Apply Formula: Use the formula: Bond Order = (Number of bonding electrons - Number of antibonding electrons) / 2.
📌 Note: The bond order for C-H is typically 1, while C-O and O-H bonds may vary based on hybridization and electron configuration.
Key Bonds in CH3OH: A Closer Look
| Bond Type | Bond Order | Significance |
|---|---|---|
| C-H | 1 | Strong, non-polar bond |
| C-O | 1 | Polar, contributes to methanol’s solubility |
| O-H | 1 | Polar, allows hydrogen bonding |

Practical Applications of CH3OH Bond Order
Understanding the bond order of CH3OH is crucial for:
- Industrial Synthesis: Optimizing production processes for methanol-based products.
- Chemical Reactions: Predicting reactivity in organic reactions.
- Material Design: Developing new materials with specific properties.
For commercial-intent visitors, this knowledge aids in selecting the right chemical suppliers, laboratory equipment, or industrial catalysts for methanol-related applications.
Checklist for Mastering CH3OH Bond Order

- Review the Lewis structure of CH3OH.
- Calculate bond orders for C-H, C-O, and O-H bonds.
- Understand the implications of bond order on molecular properties.
- Explore practical applications in your industry.
By mastering the CH3OH bond order, you’ll gain deeper insights into its chemical behavior, enabling better decision-making in both academic and industrial contexts, such as chemical engineering, organic synthesis, and material development.
What is the bond order of C-H in CH3OH?
+The bond order of C-H in CH3OH is 1, indicating a single, strong covalent bond.
How does bond order affect methanol’s solubility?
+The polar C-O and O-H bonds (both with bond order 1) allow methanol to form hydrogen bonds, enhancing its solubility in water and other polar solvents.
Can bond order predict methanol’s reactivity?
+Yes, the bond order helps predict reactivity by indicating the strength and stability of bonds, influencing how CH3OH participates in chemical reactions.



