Bond Order of NO: Understanding Nitric Oxide's Molecular Structure
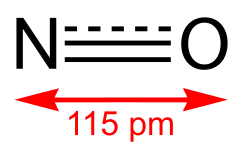
Nitric oxide (NO) is a diatomic molecule with unique chemical properties that play a crucial role in various biological and industrial processes. Understanding its bond order is essential for grasping its molecular stability and reactivity. In this post, we’ll explore the bond order of NO, its calculation, and its implications in chemistry and beyond, (molecular structure, chemical bonding, nitric oxide properties).
What is Bond Order and Why Does It Matter?
Bond order is a measure of the number of chemical bonds between a pair of atoms. It determines the stability and strength of a molecule. For nitric oxide (NO), the bond order is a key factor in understanding its behavior in biological systems and chemical reactions, (bond strength, molecular stability, chemical bonds).
Calculating the Bond Order of NO
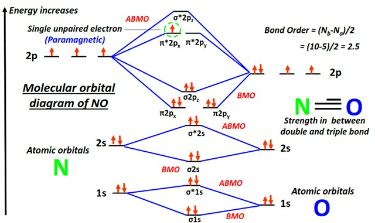
The bond order of NO can be calculated using molecular orbital theory. Here’s a step-by-step breakdown:
- Step 1: Determine the molecular orbital diagram for NO.
- Step 2: Count the number of electrons in bonding and antibonding orbitals.
- Step 3: Use the formula: Bond Order = (Number of bonding electrons - Number of antibonding electrons) / 2.
For NO, the bond order is 2.5, indicating a strong and stable bond, (molecular orbital theory, bonding electrons, antibonding electrons).
Implications of NO’s Bond Order

The bond order of 2.5 explains NO’s unique properties:
- Reactivity: NO is highly reactive due to its unpaired electron, making it a key player in biological signaling and environmental chemistry.
- Stability: Despite its reactivity, NO’s bond order ensures it remains stable under certain conditions.
| Molecule | Bond Order | Stability |
|---|---|---|
| NO | 2.5 | Moderate |
| O₂ | 2 | High |
| N₂ | 3 | Very High |

💡 Note: The bond order of NO is a critical factor in its role as a signaling molecule in biological systems.
What is the bond order of NO?
+The bond order of nitric oxide (NO) is 2.5, calculated using molecular orbital theory.
Why is NO’s bond order important?
+NO’s bond order of 2.5 explains its stability and reactivity, which are crucial in biological and chemical processes.
How does NO’s bond order compare to other molecules?
+NO’s bond order (2.5) is higher than O₂ (2) but lower than N₂ (3), reflecting its unique properties.
Understanding the bond order of NO provides valuable insights into its molecular structure and behavior. Whether in biological signaling or industrial applications, NO’s unique bond order of 2.5 makes it a fascinating molecule to study. By grasping these concepts, you’ll better appreciate the role of nitric oxide in chemistry and beyond, (molecular structure, chemical bonding, nitric oxide properties).



