Dichloromethane Boiling Point: Quick Facts & Uses

Dichloromethane, also known as methylene chloride, is a versatile solvent widely used in various industries. Its boiling point is a critical property that influences its applications, safety, and handling. In this post, we’ll explore the dichloromethane boiling point, its uses, and essential facts to help you understand this chemical better.
What is the Boiling Point of Dichloromethane?

The dichloromethane boiling point is approximately 39.6°C (103.3°F) at standard atmospheric pressure. This low boiling point makes it an excellent solvent for processes requiring quick evaporation.
| Property | Value |
|---|---|
| Boiling Point | 39.6°C (103.3°F) |
| Chemical Formula | CH2Cl2 |
| Density | 1.326 g/cm³ |
Why is the Boiling Point of Dichloromethane Important?
The low dichloromethane boiling point is crucial for its effectiveness in applications like:
- Solvent Extraction: It dissolves a wide range of organic compounds.
- Chemical Synthesis: Used in reactions requiring low-temperature conditions.
- Pharmaceuticals: Essential for purifying and isolating compounds.
📌 Note: Always handle dichloromethane in a well-ventilated area due to its volatility and potential health risks.
Key Uses of Dichloromethane

Dichloromethane’s unique properties make it invaluable in:
- Paint Stripping: Effectively removes coatings from surfaces.
- Caffeine Extraction: Widely used in the decaffeination process.
- Laboratory Applications: A common solvent for chromatography and sample preparation.
Safety Considerations When Handling Dichloromethane
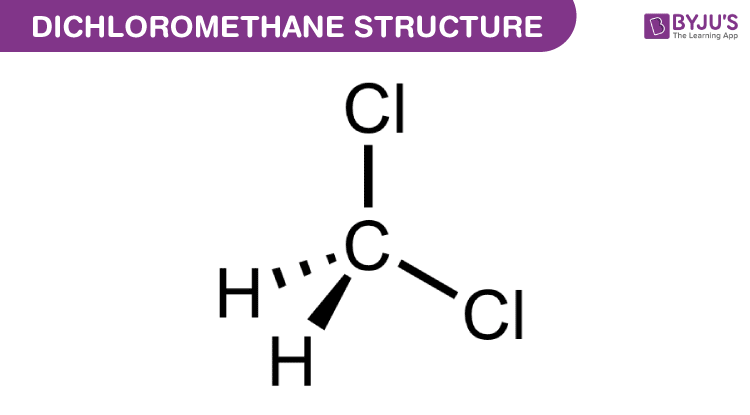
Due to its low boiling point, dichloromethane can evaporate quickly, posing inhalation risks. Key safety measures include:
- Using personal protective equipment (PPE).
- Ensuring proper ventilation.
- Storing in a cool, dry place away from heat sources.
⚠️ Note: Prolonged exposure to dichloromethane can cause health issues, including respiratory irritation and potential long-term effects.
Dichloromethane Boiling Point: A Quick Checklist
- Boiling Point: 39.6°C (103.3°F).
- Primary Uses: Solvent extraction, paint stripping, caffeine extraction.
- Safety Tips: Use PPE, ensure ventilation, avoid heat exposure.
Dichloromethane’s low boiling point makes it a highly effective solvent for various industrial and laboratory applications. However, its volatility requires careful handling to ensure safety. By understanding its properties and uses, you can leverage dichloromethane efficiently while minimizing risks.
What is the boiling point of dichloromethane?
+The boiling point of dichloromethane is approximately 39.6°C (103.3°F).
Is dichloromethane safe to use?
+Dichloromethane can be safe if handled properly with PPE, ventilation, and adherence to safety guidelines.
What are the main uses of dichloromethane?
+Dichloromethane is used in solvent extraction, paint stripping, caffeine extraction, and laboratory applications.
Related Keywords: dichloromethane uses, dichloromethane safety, solvent boiling points, chemical properties, industrial solvents.



