CH3N3 Lewis Structure Resonance: Simplified Guide

Understanding the CH3N3 Lewis Structure Resonance is crucial for anyone studying organic chemistry or chemical bonding. Methyl azide (CH3N3) is a fascinating molecule with unique resonance structures that help explain its stability and reactivity. This guide simplifies the process of drawing and understanding its Lewis structure, making it accessible for both students and professionals.
What is the CH3N3 Lewis Structure?
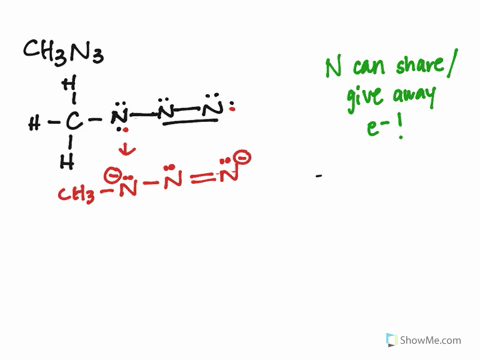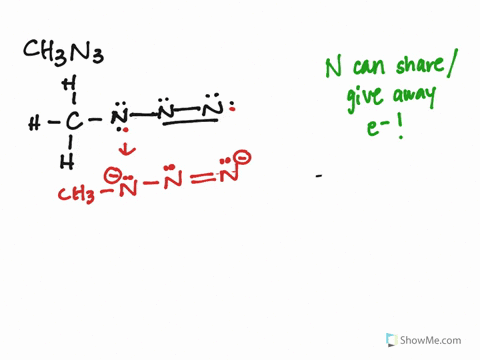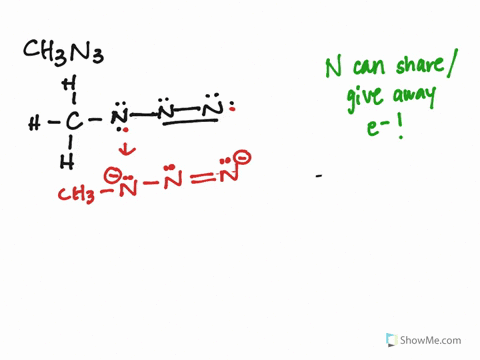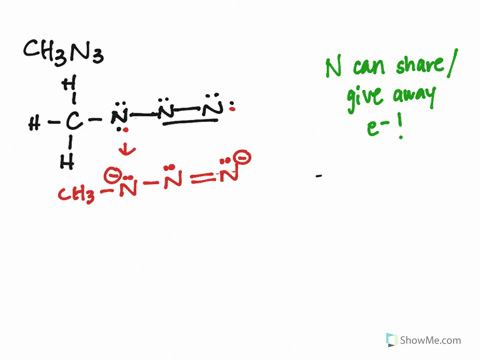
The CH3N3 Lewis structure represents the arrangement of atoms and electrons in methyl azide. It consists of a carbon atom bonded to three hydrogen atoms and an azide group (N3). The azide group is where the resonance occurs, as the double bonds can shift between the nitrogen atoms.
Step-by-Step Guide to Drawing the CH3N3 Lewis Structure
Count the Total Valence Electrons:
- Carbon ©: 4 electrons
- Hydrogen (H): 3 electrons (1 each)
- Nitrogen (N): 15 electrons (5 each)
- Total: 4 + 3 + 15 = 22 electrons
- Carbon ©: 4 electrons
Arrange the Atoms:
- Place the carbon atom in the center, bonded to three hydrogen atoms and the azide group (N3).
- Place the carbon atom in the center, bonded to three hydrogen atoms and the azide group (N3).
Form Bonds and Distribute Electrons:
- Connect the atoms with single bonds, using 6 electrons.
- Distribute the remaining 16 electrons as lone pairs, ensuring each nitrogen atom has a complete octet.
- Connect the atoms with single bonds, using 6 electrons.
Identify Resonance Structures:
- The azide group (N3) has three resonance structures, where the double bonds shift between the nitrogen atoms.
- The azide group (N3) has three resonance structures, where the double bonds shift between the nitrogen atoms.
📌 Note: Resonance structures contribute to the stability of the molecule by delocalizing electrons.
Resonance in CH3N3: Why It Matters

Resonance in CH3N3 is essential for understanding its chemical behavior. The delocalized electrons in the azide group make the molecule more stable and reactive, influencing its participation in chemical reactions.
Key Resonance Structures of CH3N3
- Structure 1: Double bond between the first and second nitrogen atoms.
- Structure 2: Double bond between the second and third nitrogen atoms.
- Structure 3: Double bond between the first and third nitrogen atoms.
| Resonance Structure | Double Bond Location |
|---|---|
| Structure 1 | N1–N2 |
| Structure 2 | N2–N3 |
| Structure 3 | N1–N3 |

Checklist for Drawing CH3N3 Lewis Structure

- [ ] Count total valence electrons (22 for CH3N3).
- [ ] Place carbon in the center and connect atoms with single bonds.
- [ ] Distribute remaining electrons as lone pairs.
- [ ] Identify and draw all resonance structures for the azide group.
For those looking to deepen their understanding, exploring Lewis structure rules and resonance theory can provide further insights.
What is the importance of resonance in CH3N3?
+Resonance in CH3N3 stabilizes the molecule by delocalizing electrons, making it more reactive and less likely to decompose spontaneously.
How many resonance structures does CH3N3 have?
+CH3N3 has three resonance structures, all involving the shifting of double bonds within the azide group (N3).
What is the role of the azide group in CH3N3?
+The azide group (N3) is responsible for the resonance in CH3N3, contributing to its stability and unique chemical properties.
In summary, mastering the CH3N3 Lewis structure resonance is key to understanding its stability and reactivity. By following the steps outlined above and exploring resonance structures, you’ll gain a solid foundation in this essential chemical concept. Whether you’re a student or a professional, this simplified guide ensures clarity and precision in your studies.
Related Keywords: Lewis structure, resonance structures, methyl azide, chemical bonding, organic chemistry.