Copper Electron Configuration: Quick Guide & Tips

Understanding copper electron configuration is essential for students, chemists, and enthusiasts alike. Copper, with its unique properties and widespread applications, holds a special place in the periodic table. Its electron configuration, Ar 3d¹⁰ 4s¹, plays a crucial role in determining its chemical behavior and physical characteristics. Whether you’re studying for an exam or exploring copper’s industrial uses, this guide provides quick insights and practical tips to master the topic.
What is Copper Electron Configuration?
Copper (Cu) is a transition metal with the atomic number 29. Its electron configuration is Ar 3d¹⁰ 4s¹, which indicates that copper has a fully filled 3d orbital and a single electron in the 4s orbital. This arrangement is slightly unusual, as one might expect the 4s electrons to be removed first. However, the 3d¹⁰ configuration is more stable due to the lower energy state of a fully filled d-orbital.
💡 Note: The stability of the 3d¹⁰ configuration is a key reason why copper exhibits this electron arrangement.
Why is Copper’s Electron Configuration Important?
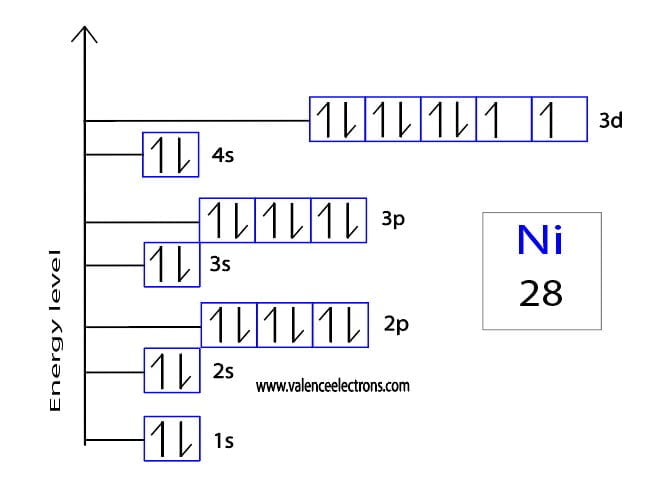
Copper’s electron configuration explains its excellent conductivity, malleability, and chemical reactivity. The single electron in the 4s orbital is loosely bound, allowing it to move freely and conduct electricity. Additionally, this configuration influences copper’s ability to form various compounds, making it valuable in industries like electronics, construction, and chemistry.
How to Remember Copper’s Electron Configuration

Memorizing electron configurations can be challenging, but here are some tips:
- Use the Periodic Table: Locate copper in the transition metal section (Group 11, Period 4).
- Noble Gas Shortcut: Start with the nearest noble gas, argon (Ar), and add the remaining electrons.
- Practice Writing: Write the configuration repeatedly to reinforce memory.
Common Applications of Copper’s Electron Configuration

Copper’s unique electron arrangement makes it ideal for:
- Electrical Wiring: High conductivity ensures efficient energy transfer.
- Alloys: Copper is used in alloys like brass and bronze for durability.
- Catalysis: Its reactivity makes it a useful catalyst in chemical reactions.
Checklist for Mastering Copper Electron Configuration

- [ ] Understand the basic electron configuration: Ar 3d¹⁰ 4s¹.
- [ ] Learn why the 3d¹⁰ configuration is more stable.
- [ ] Explore copper’s applications in industries.
- [ ] Practice writing the configuration from memory.
What is the electron configuration of copper?
+Copper's electron configuration is Ar 3d¹⁰ 4s¹.
Why does copper have an unusual electron configuration?
+Copper’s 3d¹⁰ 4s¹ configuration is more stable due to the lower energy state of a fully filled 3d orbital.
How does copper’s electron configuration affect its properties?
+It enhances conductivity, malleability, and reactivity, making copper valuable in various industries.
In summary, copper’s electron configuration, Ar 3d¹⁰ 4s¹, is a fascinating aspect of its chemistry. By understanding this arrangement, you can appreciate its stability, properties, and applications. Use the checklist and tips provided to solidify your knowledge and explore related topics like transition metals, electron stability, periodic trends, and copper compounds.



