Electrons in Oxygen: Unlocking Atomic Secrets
<!DOCTYPE html>
Oxygen, the life-sustaining element, is more than just a component of the air we breathe. At its core, oxygen’s unique properties stem from its electron configuration. Understanding electrons in oxygen unlocks secrets of chemistry, biology, and even industrial applications. This post delves into the fascinating world of oxygen’s atomic structure, its electron behavior, and why it matters in both scientific and practical contexts.
The Electron Configuration of Oxygen
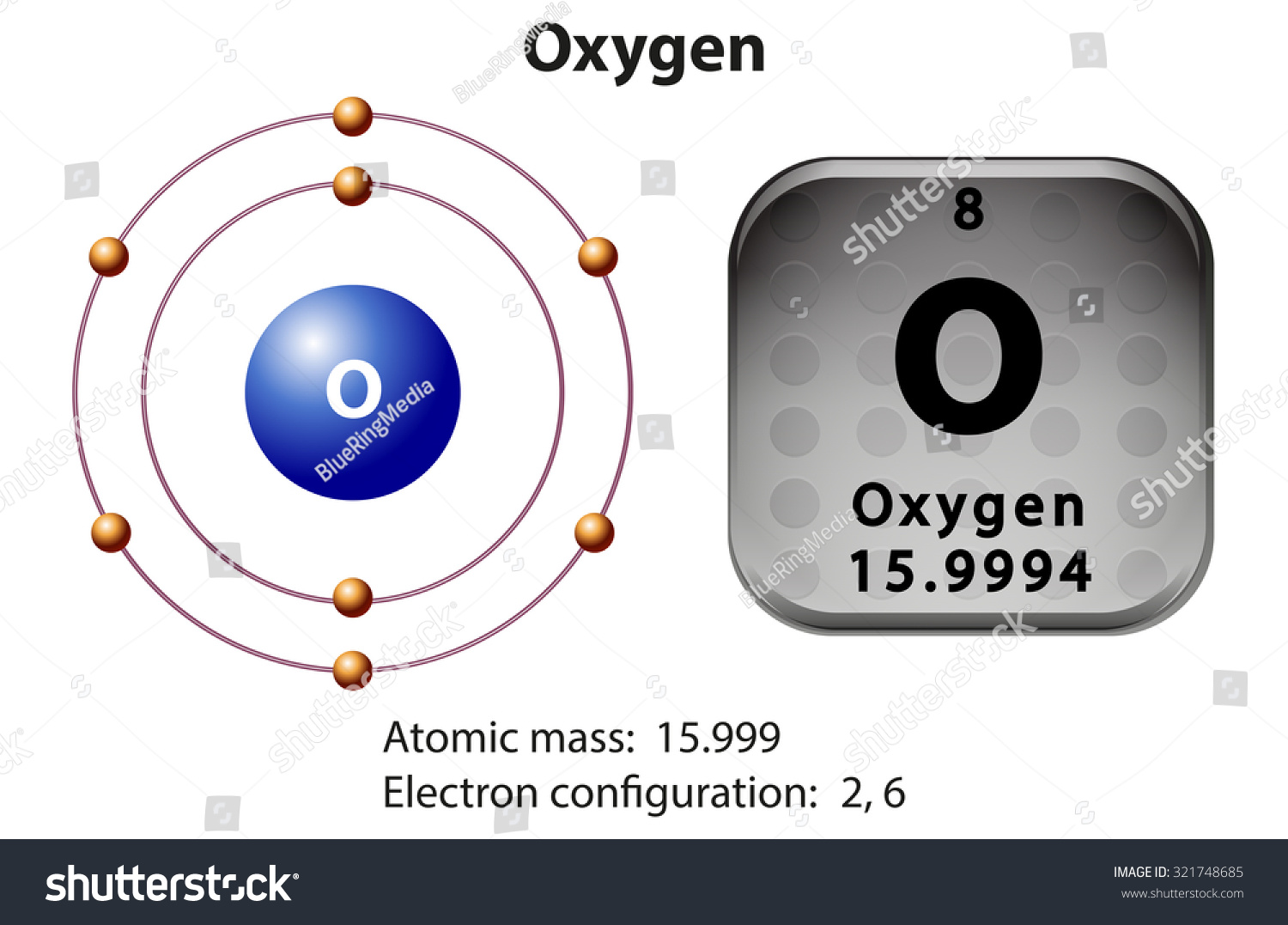
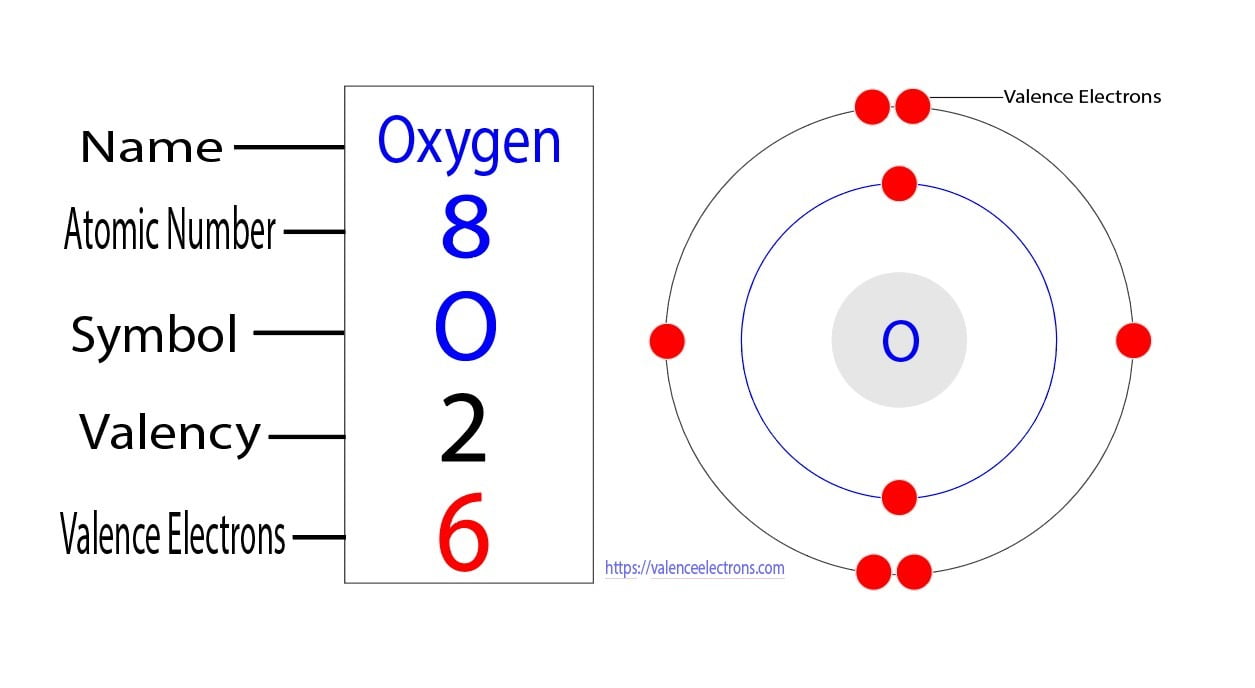
Oxygen, with its atomic number 8, has a simple yet intriguing electron arrangement. Its electron configuration is 1s² 2s² 2p⁴, meaning it has 8 electrons distributed across two energy levels. The 2p orbital, partially filled with four electrons, is key to oxygen’s reactivity and bonding capabilities. This configuration explains why oxygen readily forms compounds with other elements, such as in water (H₂O) or carbon dioxide (CO₂).
Why Oxygen’s Electrons Matter
The behavior of electrons in oxygen is crucial for several reasons:
- Chemical Reactivity: Oxygen’s partially filled 2p orbital makes it highly reactive, allowing it to form oxides with metals and participate in combustion reactions.
- Biological Significance: In cellular respiration, oxygen accepts electrons in the electron transport chain, enabling energy production in living organisms.
- Industrial Applications: Understanding oxygen’s electron behavior is vital for processes like steel production, where oxygen is used to remove impurities.
Oxygen’s Role in Chemical Bonding

Oxygen’s electrons play a central role in forming chemical bonds. It typically forms covalent bonds by sharing electrons with other atoms, as seen in O₂ molecules. However, in ionic compounds like sodium oxide (Na₂O), oxygen gains electrons to form oxide ions (O²⁻). This versatility highlights the importance of oxygen’s electron configuration in chemistry.
Oxygen in Oxidation Reactions
Oxygen is a powerful oxidizing agent, readily accepting electrons from other substances. This property is evident in:
- Combustion reactions, where oxygen reacts with fuels to release energy.
- Rusting of iron, where oxygen oxidizes iron to form iron oxide.
📌 Note: Oxygen’s ability to accept electrons makes it a key player in redox reactions, essential in both natural and industrial processes.
Practical Applications of Oxygen’s Electron Behavior

The study of electrons in oxygen has led to numerous practical applications:
| Application | Description |
|---|---|
| Medicine | Oxygen therapy relies on its electron configuration to support respiration in patients. |
| Environmental Science | Understanding oxygen’s role in ozone formation helps combat air pollution. |
| Energy Production | Fuel cells utilize oxygen’s electron-accepting properties to generate electricity. |

Checklist for Understanding Oxygen’s Electrons
- Learn oxygen’s electron configuration: 1s² 2s² 2p⁴.
- Explore its role in chemical bonding and redox reactions.
- Study practical applications in medicine, industry, and energy.
In summary, the electrons in oxygen are the cornerstone of its reactivity and versatility. From sustaining life to driving industrial processes, understanding oxygen’s atomic secrets opens doors to innovation and discovery. Whether you’re a student, researcher, or industry professional, grasping these concepts is essential for unlocking oxygen’s full potential. (electron configuration, chemical bonding, oxidation reactions)
What is oxygen’s electron configuration?
+Oxygen’s electron configuration is 1s² 2s² 2p⁴, with 8 electrons distributed across two energy levels.
Why is oxygen highly reactive?
+Oxygen’s partially filled 2p orbital allows it to readily share or accept electrons, making it highly reactive.
How does oxygen contribute to energy production?
+In cellular respiration, oxygen accepts electrons in the electron transport chain, enabling ATP production in living organisms.



