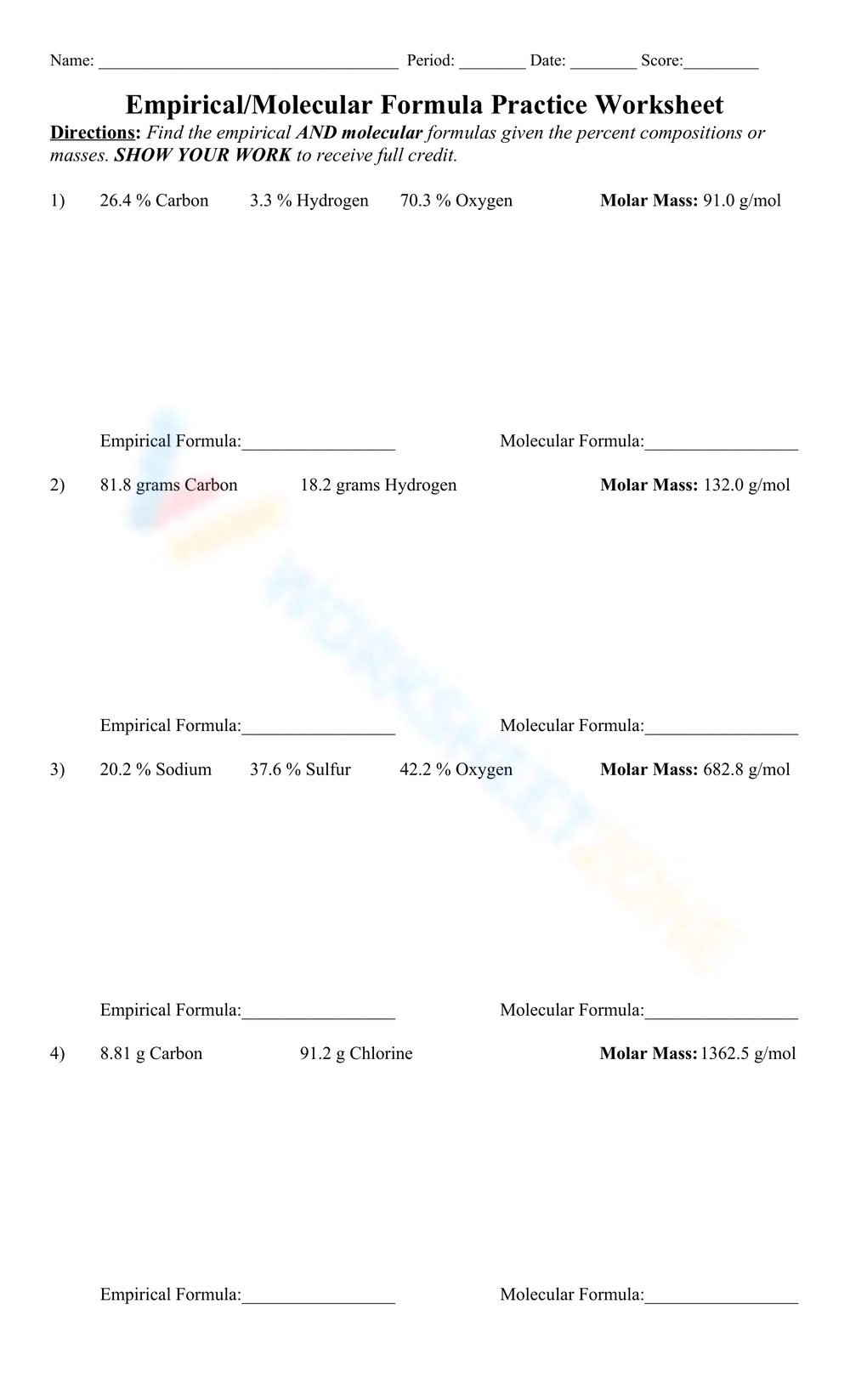
Empirical Formula Worksheet: Practice Problems & Answers

<!DOCTYPE html>
Mastering the empirical formula is crucial for chemistry students and enthusiasts alike. Whether you’re preparing for exams or simply looking to enhance your understanding, our Empirical Formula Worksheet provides practical problems and detailed answers to guide you through the process. This resource is designed to help you calculate empirical formulas with confidence, ensuring you grasp the fundamental concepts of chemical composition.
Understanding the Empirical Formula
The empirical formula represents the simplest whole-number ratio of elements in a compound. It’s a foundational concept in chemistry that helps identify the basic structure of a substance. By learning how to derive empirical formulas, you’ll gain insights into the molecular makeup of various compounds.
📌 Note: Always ensure your calculations are based on accurate mass percentages or compositional data.
Step-by-Step Guide to Solving Empirical Formula Problems

Step 1: Gather Compositional Data
Start by obtaining the mass percentages of each element in the compound. For example, if a compound contains 40% carbon, 6.7% hydrogen, and 53.3% oxygen, these values are your starting point.
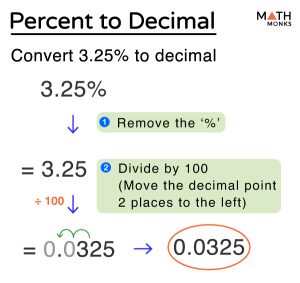
Step 2: Convert Percentages to Grams
Assume you have 100 grams of the compound. Convert the percentages directly into grams. For instance, 40% carbon becomes 40 grams, 6.7% hydrogen becomes 6.7 grams, and 53.3% oxygen becomes 53.3 grams.
Step 3: Calculate Moles of Each Element
Use the molar mass of each element to convert grams to moles. For carbon (12 g/mol), hydrogen (1 g/mol), and oxygen (16 g/mol), the calculations would be:
- Carbon: 40 g ÷ 12 g/mol = 3.33 moles
- Hydrogen: 6.7 g ÷ 1 g/mol = 6.7 moles
- Oxygen: 53.3 g ÷ 16 g/mol = 3.33 moles
Step 4: Determine the Simplest Ratio
Divide each mole value by the smallest number of moles to find the simplest whole-number ratio. In this case, dividing by 3.33 gives:
- Carbon: 3.33 ÷ 3.33 = 1
- Hydrogen: 6.7 ÷ 3.33 ≈ 2
- Oxygen: 3.33 ÷ 3.33 = 1
The empirical formula is CH₂O.
Practice Problems with Answers

Test your skills with these practice problems. Solutions are provided to help you verify your answers.
| Problem | Composition | Empirical Formula |
|---|---|---|
| 1 | 52.1% C, 13.1% H, 34.8% O | C₂H₄O |
| 2 | 40.0% C, 6.7% H, 53.3% O | CH₂O |
| 3 | 28.6% K, 1.9% H, 22.5% P, 47.0% O | K₂HPO₄ |

Checklist for Mastering Empirical Formulas

- Always start with accurate compositional data.
- Convert percentages to grams assuming a 100g sample.
- Calculate moles using molar masses.
- Determine the simplest whole-number ratio.
- Practice regularly with diverse problems.
By following these steps and practicing with our worksheet, you’ll become proficient in calculating empirical formulas. Whether you’re a student or a professional, this skill is essential for understanding chemical compounds, (empirical formula worksheet, chemistry practice, chemical composition)
What is an empirical formula?
+An empirical formula represents the simplest whole-number ratio of elements in a compound.
How do I convert mass percentages to moles?
+Assume a 100g sample, convert percentages to grams, and divide by the molar mass of each element.
Why is the empirical formula important?
+It helps identify the basic structure and composition of a compound, which is essential in chemistry.