H2O Lewis Dot Structure: Simple Visual Guide
Understanding the H2O Lewis Dot Structure is essential for anyone studying chemistry, especially when it comes to grasping molecular bonding and electron distribution. Water (H₂O) is one of the most fundamental molecules, and its Lewis structure provides a clear visual representation of how atoms share electrons. This guide will walk you through the process of drawing the H2O Lewis Dot Structure step-by-step, ensuring you understand the concept thoroughly.
What is the H2O Lewis Dot Structure?

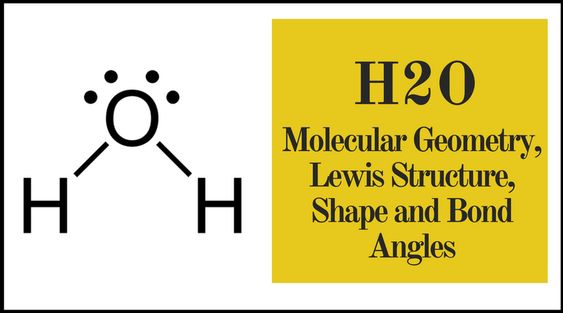
The H2O Lewis Dot Structure is a diagram that shows the bonding between oxygen and hydrogen atoms in a water molecule, along with the distribution of valence electrons. It helps visualize the covalent bonds and lone pairs of electrons in the molecule.
Why is the H2O Lewis Dot Structure Important?
- Understanding Molecular Geometry: It provides insights into the shape of the water molecule.
- Electron Distribution: It highlights how electrons are shared or distributed among atoms.
- Chemical Properties: It helps explain water’s unique chemical behavior, such as its polarity.
📌 Note: The Lewis structure is a foundational concept in chemistry, crucial for predicting molecular behavior.
Step-by-Step Guide to Drawing the H2O Lewis Dot Structure

Step 1: Determine the Total Number of Valence Electrons
Oxygen has 6 valence electrons, and each hydrogen atom has 1. Since H₂O has one oxygen and two hydrogen atoms:
Total valence electrons = 6 (O) + 2(1) (H) = 8 electrons.
Step 2: Identify the Central Atom
Oxygen is the central atom because it is more electronegative and can form more bonds.
Step 3: Connect Atoms with Single Bonds
Draw single bonds between the oxygen atom and each hydrogen atom. This uses up 4 electrons (2 bonds).
Step 4: Distribute Remaining Electrons
Place the remaining 4 electrons as lone pairs on the oxygen atom to satisfy the octet rule.
Step 5: Verify the Octet Rule
Ensure each atom has a complete octet (except hydrogen, which needs only 2 electrons).
| Atom | Electrons in Bonds | Lone Pairs | Total Electrons |
|---|---|---|---|
| Oxygen | 4 | 4 | 8 |
| Hydrogen | 2 | 0 | 2 |

📌 Note: Hydrogen only needs 2 electrons to be stable, while oxygen requires 8.
Key Takeaways and Checklist

- Valence Electrons: Oxygen (6), Hydrogen (1 each).
- Central Atom: Oxygen.
- Bonding: Two single bonds between O and H.
- Lone Pairs: Four electrons on oxygen.
- Octet Rule: Satisfied for oxygen; hydrogen has a duet.
Checklist for Drawing H2O Lewis Dot Structure:
1. Count total valence electrons.
2. Place oxygen as the central atom.
3. Draw single bonds to hydrogen atoms.
4. Add lone pairs to oxygen.
5. Verify the octet rule.
Final Thoughts

Mastering the H2O Lewis Dot Structure is a crucial step in understanding molecular chemistry. By following this simple visual guide, you can easily draw the structure and apply the same principles to other molecules. Remember, practice makes perfect, so try drawing Lewis structures for other compounds to reinforce your learning.
What is the H2O Lewis Dot Structure?
+The H2O Lewis Dot Structure shows the bonding between oxygen and hydrogen atoms, with oxygen as the central atom, two single bonds, and two lone pairs on oxygen.
How many valence electrons does H2O have?
+H2O has a total of 8 valence electrons: 6 from oxygen and 1 from each hydrogen atom.
Why is oxygen the central atom in H2O?
+Oxygen is the central atom because it is more electronegative and can form more bonds compared to hydrogen.
Related Keywords: Lewis structure, molecular geometry, covalent bonds, electron distribution, water molecule, chemical properties.