HCC-H Molecular Geometry: Shape & Bonding Explained
Understanding the molecular geometry of compounds like HCC-H is crucial for grasping their chemical behavior and applications. HCC-H, also known as ethyne or acetylene, is a simple yet fascinating molecule with a linear shape. This blog explores the HCC-H molecular geometry, its bonding characteristics, and why it matters in chemistry. Whether you're a student, researcher, or industry professional, this guide provides clear insights into the structure and properties of HCC-H, molecular geometry, chemical bonding.
What is HCC-H Molecular Geometry?

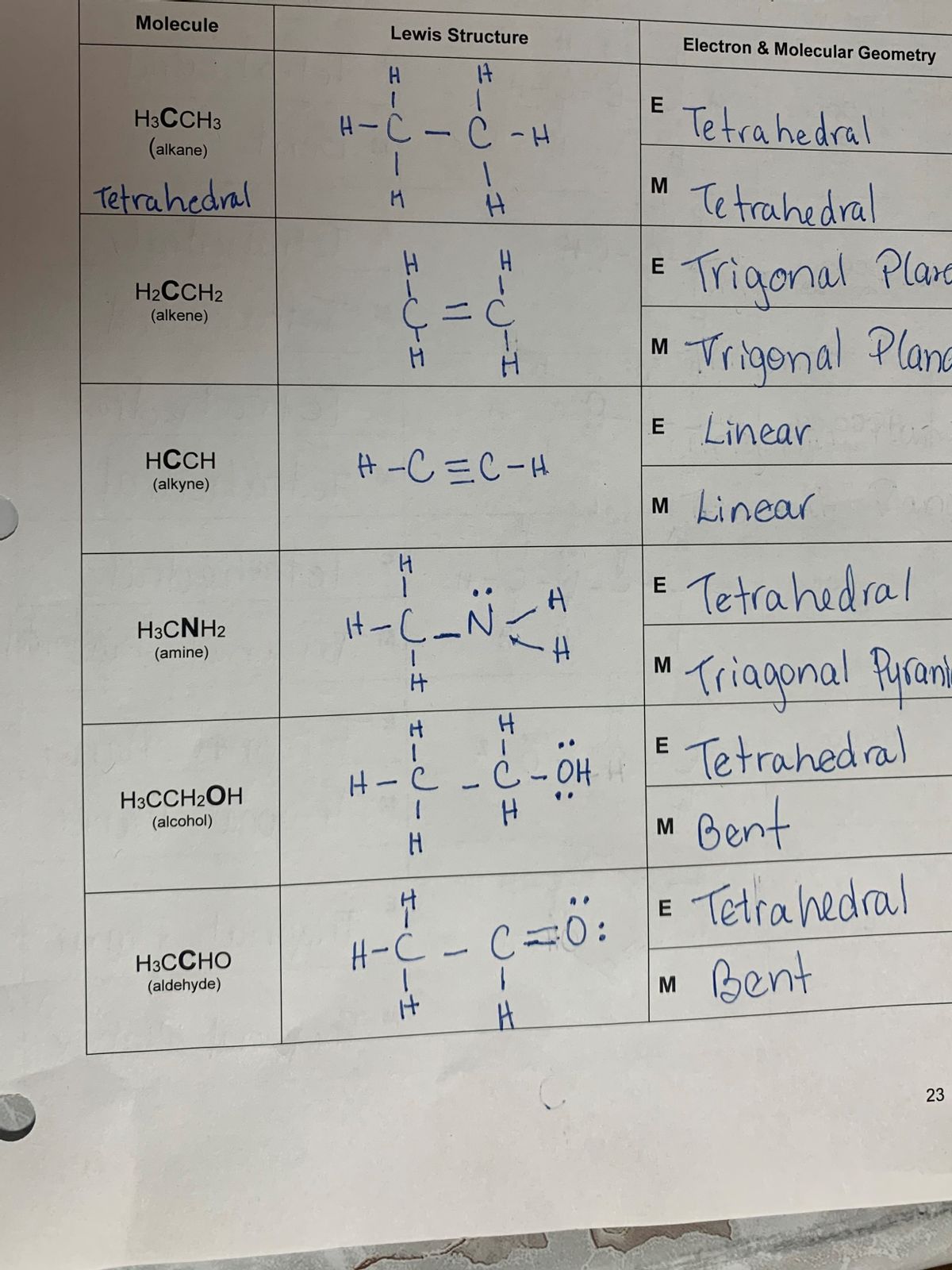
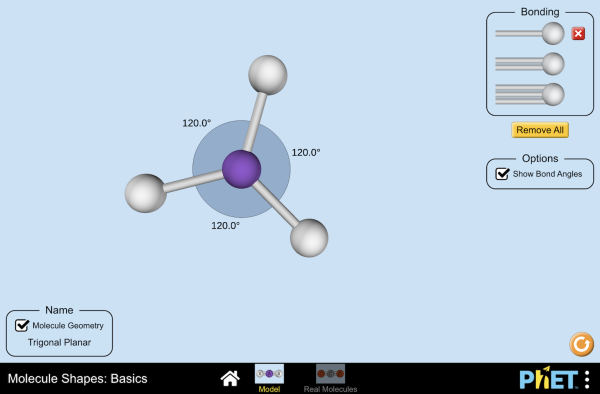
HCC-H, or ethyne, consists of two carbon atoms and two hydrogen atoms. Its molecular geometry is linear, meaning all atoms lie on a straight line. This shape is determined by the arrangement of its atoms and the type of bonds they form. The linear structure is a result of the sp hybridization of the carbon atoms, which allows for the formation of a triple bond between them. Understanding this geometry is essential for predicting its reactivity and applications in organic chemistry, chemical bonding, molecular structure.
Bonding in HCC-H: A Closer Look
The bonding in HCC-H is characterized by a carbon-carbon triple bond, which consists of one sigma (σ) bond and two pi (π) bonds. This triple bond is responsible for the molecule’s linear shape and high bond energy. The two hydrogen atoms are attached to the carbon atoms via single sigma bonds. Below is a breakdown of the bonding in HCC-H:
| Bond Type | Atoms Involved | Bond Order |
|---|---|---|
| Sigma (σ) | C-C, C-H | 1 |
| Pi (π) | C-C | 2 |

📌 Note: The triple bond in HCC-H makes it a highly reactive molecule, often used in welding and as a starting material for synthesizing other organic compounds.
Why HCC-H Molecular Geometry Matters

The linear geometry of HCC-H influences its physical and chemical properties. For instance, its shape allows for efficient packing in solid form, affecting its melting and boiling points. Additionally, the triple bond contributes to its high energy content, making it a valuable fuel source. Key applications of HCC-H include:
- Welding and cutting metals
- Synthesis of polymers and pharmaceuticals
- Use as a fuel in specialized applications
Key Takeaways: HCC-H Molecular Geometry
- HCC-H has a linear molecular geometry due to sp hybridization.
- It features a carbon-carbon triple bond (1 σ and 2 π bonds).
- Its shape and bonding determine its reactivity and applications.
In summary, the HCC-H molecular geometry is a prime example of how atomic arrangement and bonding dictate a molecule's properties. Its linear shape and triple bond make it a versatile and reactive compound, essential in various industries. By understanding its structure, chemists can harness its potential for innovative applications. Whether you're studying chemistry or working in the field, HCC-H offers valuable insights into molecular design and functionality, molecular geometry, chemical bonding.
What is the molecular geometry of HCC-H?
+HCC-H has a linear molecular geometry due to the sp hybridization of its carbon atoms.
What type of bond is present in HCC-H?
+HCC-H contains a carbon-carbon triple bond, consisting of one sigma (σ) and two pi (π) bonds.
Why is HCC-H important in chemistry?
+HCC-H is important due to its high reactivity, making it useful in welding, organic synthesis, and as a fuel source.



