HCN Lewis Structure: A Simple Guide to Dot Structure Drawing
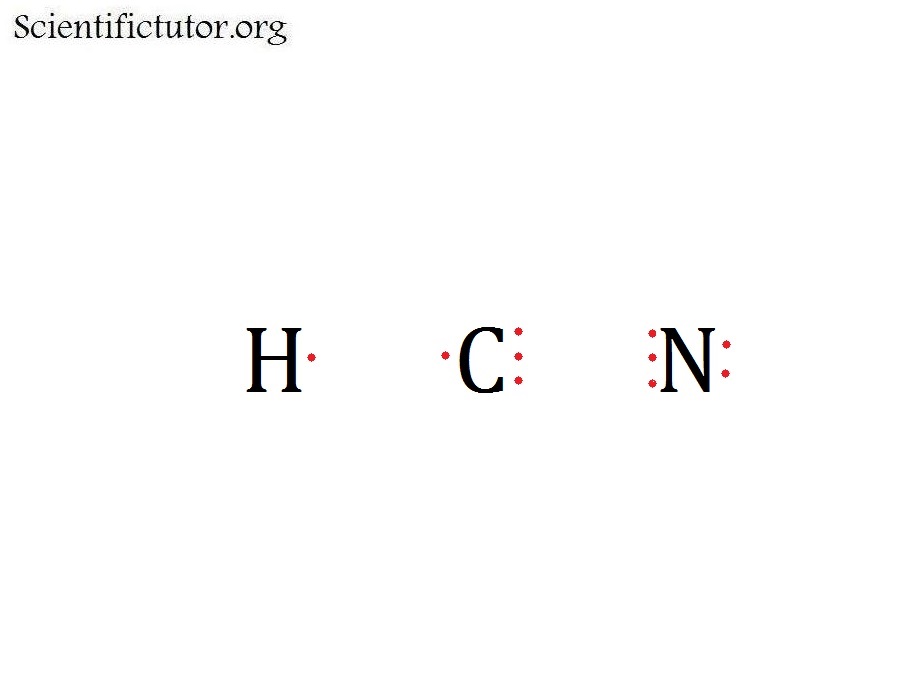
Understanding the HCN Lewis structure is essential for anyone studying chemistry, especially when dealing with molecular geometry and bonding. Hydrogen Cyanide (HCN) is a polar molecule with a linear shape, and mastering its Lewis dot structure helps in predicting its properties and reactivity. This guide provides a step-by-step approach to drawing the HCN Lewis structure, ensuring clarity and precision for both students and professionals. Whether you're preparing for an exam or working on a research project, this tutorial will simplify the process and enhance your understanding of molecular structures.
Step-by-Step Guide to Drawing the HCN Lewis Structure
Drawing the HCN Lewis structure involves a systematic approach to represent the arrangement of atoms and valence electrons. Follow these steps to create an accurate dot structure:
Step 1: Determine the Total Number of Valence Electrons
Hydrogen (H) has 1 valence electron, Carbon © has 4 valence electrons, and Nitrogen (N) has 5 valence electrons. Calculate the total:
- H: 1
- C: 4
- N: 5
Total valence electrons = 1 + 4 + 5 = 10.
Step 2: Identify the Central Atom
In HCN, Carbon © is the central atom because it is less electronegative than Nitrogen and can form multiple bonds. (HCN Lewis structure, Lewis dot structure, molecular geometry)
Step 3: Connect Atoms with Single Bonds
Place Carbon in the center and connect Hydrogen and Nitrogen with single bonds. This uses up 4 electrons (2 bonds), leaving 6 electrons to be distributed.
Step 4: Complete the Octet Rule
Nitrogen needs 6 electrons to complete its octet. Place the remaining 4 electrons as lone pairs on Nitrogen. Hydrogen already has its full shell with the single bond.
Step 5: Check for Stability
Convert one lone pair on Nitrogen into a double bond with Carbon to satisfy the octet rule for Carbon. This results in a triple bond between Carbon and Nitrogen, with Nitrogen having one lone pair.
💡 Note: The HCN Lewis structure is linear due to the triple bond, which contributes to its polarity.
Understanding the HCN Molecular Geometry

The HCN molecular geometry is linear, with a bond angle of approximately 180 degrees. This geometry arises from the arrangement of atoms and the presence of a triple bond between Carbon and Nitrogen. The molecule is polar due to the electronegativity difference between Nitrogen and Hydrogen.
| Property | Description |
|---|---|
| Shape | Linear |
| Bond Angle | 180 degrees |
| Polarity | Polar |

Checklist for Drawing the HCN Lewis Structure

Use this checklist to ensure accuracy when drawing the HCN Lewis structure:
- Calculate total valence electrons: 10.
- Place Carbon as the central atom.
- Connect atoms with single bonds initially.
- Complete the octet for Nitrogen with lone pairs.
- Convert lone pairs to a triple bond for stability.
- Verify linear geometry and polarity.
Mastering the HCN Lewis structure is crucial for understanding its molecular geometry and properties. By following the steps outlined in this guide, you can accurately draw the dot structure and predict its behavior in chemical reactions. Remember, practice is key to becoming proficient in Lewis structure drawing. (HCN molecular geometry, Lewis dot structure, valence electrons)
What is the molecular geometry of HCN?
+
The molecular geometry of HCN is linear due to the triple bond between Carbon and Nitrogen.
How many valence electrons are in HCN?
+
HCN has a total of 10 valence electrons (1 from H, 4 from C, and 5 from N).
Why is HCN a polar molecule?
+
HCN is polar due to the electronegativity difference between Nitrogen and Hydrogen, creating a net dipole moment.



