How Many Protons Are in Potassium?
Potassium is an essential mineral that plays a crucial role in maintaining bodily functions, from nerve impulses to muscle contractions. But have you ever wondered, "How many protons are in potassium?" Understanding the atomic structure of potassium not only satisfies curiosity but also sheds light on its chemical behavior and importance in various fields, including biology, chemistry, and nutrition. Let’s dive into the details to uncover the answer and explore why it matters.
Understanding Potassium’s Atomic Structure
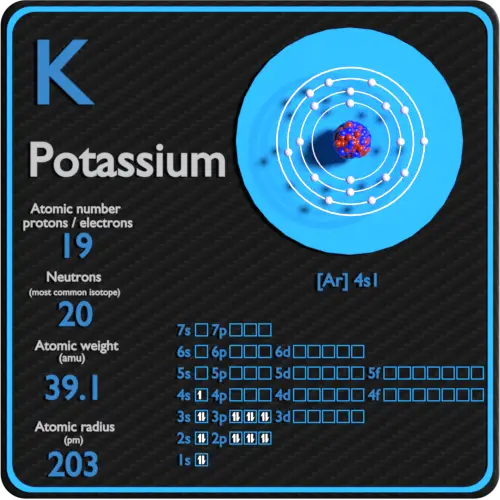
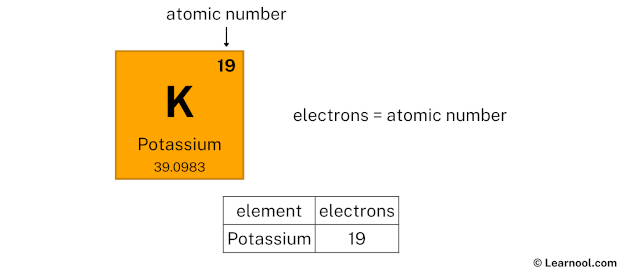
Potassium, represented by the symbol K on the periodic table, is a chemical element with the atomic number 19. This means that a neutral potassium atom contains 19 protons in its nucleus. Protons are positively charged subatomic particles that define the element’s identity. Alongside protons, potassium atoms have 19 electrons orbiting the nucleus, balancing the charge, and typically 20 neutrons (though this can vary in isotopes).
📌 Note: The number of protons in an atom is its atomic number, which uniquely identifies the element. For potassium, this number is always 19.
Why the Number of Protons in Potassium Matters
The number of protons in potassium determines its chemical properties and behavior. For instance, potassium’s reactivity is influenced by its single valence electron, which is easily lost to form a stable ion (K⁺). This property makes potassium vital in biological systems, such as regulating fluid balance and nerve function.
In commercial applications, potassium compounds like potassium chloride are used in fertilizers, pharmaceuticals, and food additives. Understanding its atomic structure helps scientists and industries optimize its use effectively.
| Property | Value |
|---|---|
| Atomic Number | 19 |
| Protons | 19 |
| Electrons | 19 |
| Neutrons (most common isotope) | 20 |

Potassium in Everyday Life
Potassium’s atomic structure isn’t just a theoretical concept—it has practical implications. In nutrition, potassium-rich foods like bananas, spinach, and potatoes support heart health and muscle function. Industrially, potassium compounds are used in soaps, glass manufacturing, and as a salt substitute in low-sodium diets.
For those with commercial interests, potassium’s unique properties make it a valuable resource in agriculture and healthcare, driving demand for potassium-based products worldwide.
Key Takeaways Checklist:
- Potassium has 19 protons, defining its atomic identity.
- Its single valence electron makes it highly reactive and useful in biology and industry.
- Potassium is essential for health and widely used in commercial applications.
In summary, potassium’s 19 protons are the foundation of its unique properties and applications. Whether you’re studying chemistry, focusing on nutrition, or exploring industrial uses, understanding its atomic structure is key. From maintaining bodily functions to enhancing agricultural productivity, potassium’s role is undeniable. (potassium atomic structure, potassium uses, potassium in nutrition)
How many protons are in potassium?
+
Potassium has 19 protons, as indicated by its atomic number 19.
Why is potassium important in biology?
+
Potassium is crucial for nerve function, muscle contractions, and maintaining fluid balance in cells.
What are common uses of potassium in industry?
+
Potassium is used in fertilizers, pharmaceuticals, glass manufacturing, and as a salt substitute.


