Uncovering Alkene Secrets with IR Spectroscopy

In the world of organic chemistry, understanding the structure of alkenes is crucial for both research and industrial applications. Infrared (IR) spectroscopy stands out as a powerful tool for uncovering the secrets of these compounds. By analyzing the unique IR spectra, chemists can identify functional groups, determine bond strengths, and even predict reactivity. Whether you're a student, researcher, or industry professional, mastering IR spectroscopy for alkenes can significantly enhance your analytical skills. In this post, we’ll explore the fundamentals, techniques, and practical tips for using IR spectroscopy to study alkenes, ensuring you gain valuable insights into their molecular structure. (IR spectroscopy for alkenes, alkene analysis, organic chemistry)
Understanding Alkenes and IR Spectroscopy Basics

Alkenes, characterized by their carbon-carbon double bonds, exhibit distinct properties that make them fascinating subjects for study. IR spectroscopy works by detecting vibrational transitions in molecules, producing spectra that reveal specific bond types and functional groups. The key to interpreting these spectra lies in recognizing the absorption bands associated with alkenes, typically found between 1600–1700 cm⁻¹, known as the C=C stretching region. (alkene structure, IR spectroscopy basics, C=C stretching)
Key IR Spectral Features of Alkenes

When analyzing alkenes using IR spectroscopy, focus on the following critical features:
- C=C Stretching Region (1600–1700 cm⁻¹): The most prominent indicator of alkenes.
- C-H Stretching (3000–3100 cm⁻¹): Reveals the presence of alkyl groups attached to the double bond.
- Bending Vibrations (1000–1500 cm⁻¹): Provides additional structural information.
📌 Note: The exact position of the C=C stretch can vary depending on substitution and conjugation. (IR spectral analysis, alkene identification, C-H stretching)
Practical Steps for Analyzing Alkenes with IR Spectroscopy
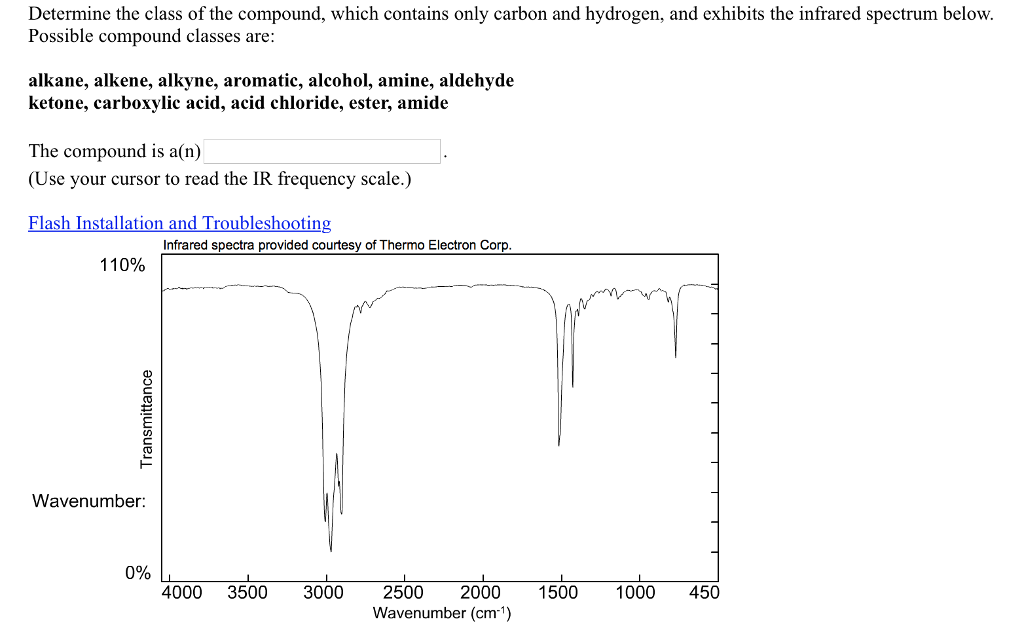
To effectively analyze alkenes using IR spectroscopy, follow these steps:
- Prepare Your Sample: Ensure the sample is pure and free from contaminants.
- Record the Spectrum: Use an IR spectrometer to obtain a clear spectrum.
- Identify Key Peaks: Focus on the C=C and C-H stretching regions.
- Compare with Reference Data: Use databases or literature to confirm your findings.
By systematically following these steps, you can confidently identify and characterize alkenes. (IR spectroscopy techniques, sample preparation, peak identification)
Advanced Tips for IR Spectroscopy of Alkenes
For more advanced analysis, consider these tips:
- Look for Conjugation Effects: Conjugated alkenes may show shifts in the C=C stretching region.
- Analyze Substituent Effects: Electron-donating or -withdrawing groups can alter peak positions.
- Use Derivatization: Convert alkenes into more detectable forms if necessary.
These advanced techniques can provide deeper insights into the structure and properties of alkenes. (conjugated alkenes, substituent effects, derivatization techniques)
Checklist for IR Spectroscopy of Alkenes

- ✅ Prepare a pure sample for analysis.
- ✅ Identify the C=C stretching region (1600–1700 cm⁻¹).
- ✅ Analyze C-H stretching and bending vibrations.
- ✅ Compare results with reference spectra.
- ✅ Consider conjugation and substituent effects for advanced analysis.
IR spectroscopy is an indispensable tool for uncovering the secrets of alkenes. By understanding the key spectral features and following practical steps, you can accurately identify and characterize these compounds. Whether you're a beginner or an experienced chemist, mastering IR spectroscopy will undoubtedly enhance your analytical capabilities. Start applying these techniques today and take your alkene analysis to the next level. (IR spectroscopy applications, alkene characterization, analytical chemistry)
What is the characteristic IR peak for alkenes?
+
The characteristic IR peak for alkenes is observed in the C=C stretching region, typically between 1600–1700 cm⁻¹.
How does conjugation affect IR spectra of alkenes?
+
Conjugation can cause a shift in the C=C stretching peak, often resulting in a lower wavenumber due to delocalization of electrons.
Can IR spectroscopy distinguish between different alkenes?
+
Yes, by analyzing peak positions, intensities, and additional vibrations, IR spectroscopy can help distinguish between different alkene structures.



