IR Spectrum Nitrile: Key Peaks and Analysis Tips
Infrared (IR) spectroscopy is a powerful tool for identifying functional groups in organic compounds, and nitriles are no exception. The IR spectrum of a nitrile compound reveals distinctive peaks that provide valuable insights into its structure. Understanding these key peaks and mastering analysis techniques can significantly enhance your ability to interpret spectra accurately. Whether you're a student, researcher, or industry professional, this guide will walk you through the essentials of IR spectrum nitrile analysis, ensuring you grasp the fundamentals and apply them effectively. (IR spectroscopy, nitrile compounds, functional group identification)
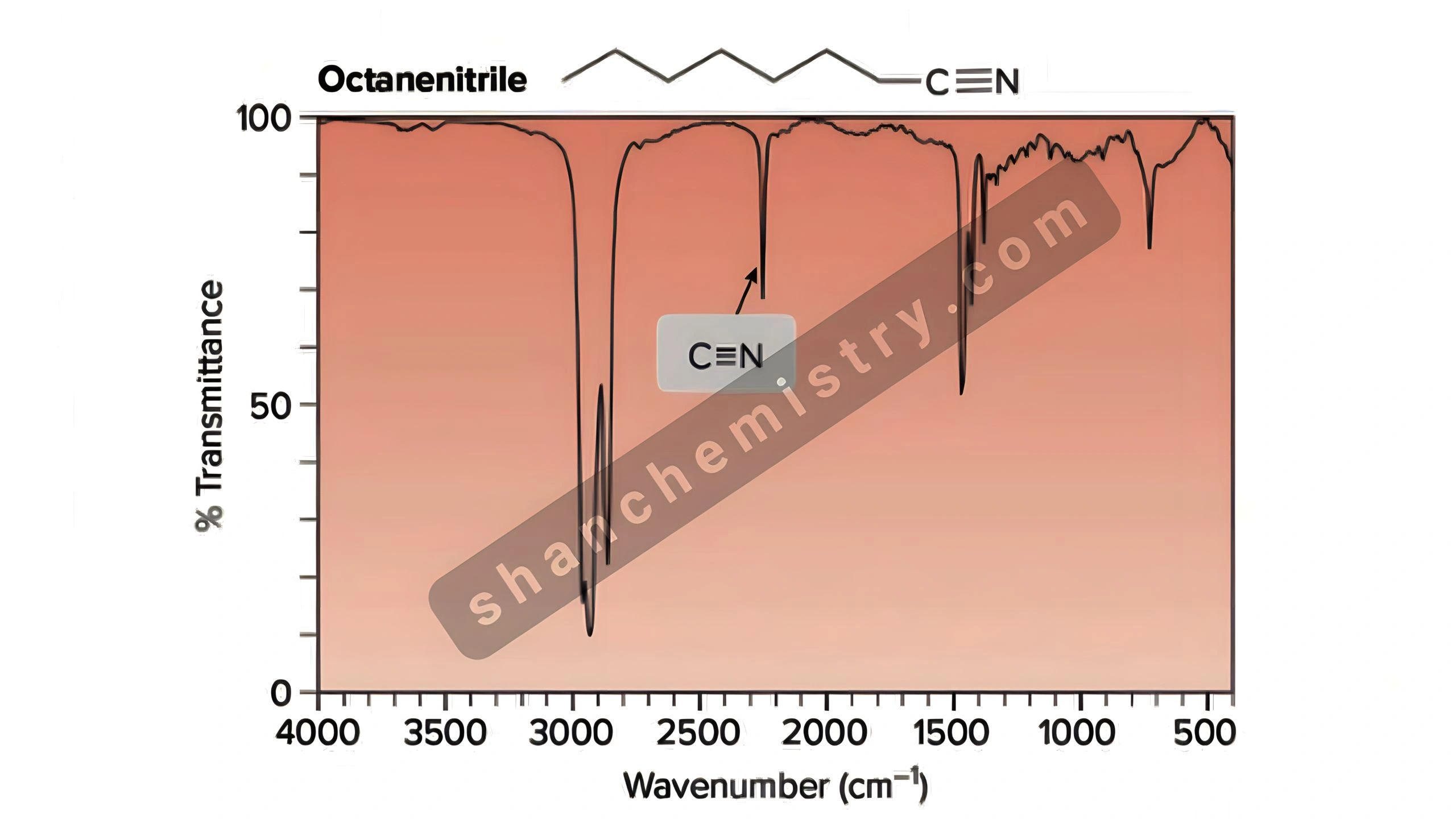
Key Peaks in IR Spectrum of Nitriles

Nitriles are characterized by the presence of a cyano group (C≡N), which gives rise to specific absorption bands in the IR spectrum. Here are the most important peaks to look for:
- C≡N Stretch: 2210–2260 cm-1 – This is the most prominent peak in the IR spectrum of nitriles. It is sharp and intense, making it a reliable indicator of the cyano group.
- C-N Stretch: 1400–1500 cm-1 – This peak is less intense but can provide additional confirmation of the nitrile group’s presence.
Understanding these peaks is crucial for nitrile functional group identification and distinguishing nitriles from other compounds. (cyano group, IR absorption bands, nitrile peaks)
Analysis Tips for IR Spectrum Nitrile

Interpreting the IR spectrum of nitriles requires attention to detail and a systematic approach. Here are some expert tips to improve your analysis:
- Baseline Correction – Ensure the baseline is properly corrected to avoid misinterpretation of peak positions.
- Resolution Optimization – Use high-resolution settings to clearly distinguish the sharp C≡N stretch peak from other nearby absorptions.
- Comparison with Standards – Compare your spectrum with reference spectra of known nitriles to validate your findings.
By following these tips, you can enhance the accuracy of your IR spectrum analysis and confidently identify nitrile compounds. (IR spectrum interpretation, baseline correction, resolution optimization)
Checklist for IR Spectrum Nitrile Analysis

To ensure a thorough and accurate analysis, use this checklist:
- Identify the C≡N stretch peak between 2210–2260 cm-1.
- Look for the C-N stretch peak in the 1400–1500 cm-1 region.
- Compare the spectrum with known nitrile reference spectra.
- Verify baseline correction and resolution settings.
This checklist will streamline your workflow and improve the reliability of your results. (IR spectrum checklist, nitrile analysis, peak identification)
📌 Note: The C≡N stretch peak is highly sensitive to substituents, so slight shifts may occur depending on the molecular environment.
Mastering the IR spectrum of nitriles is essential for anyone working in organic chemistry or analytical chemistry. By focusing on the key peaks and applying the analysis tips provided, you can confidently identify nitrile compounds and interpret their spectra with precision. Remember, practice and attention to detail are key to becoming proficient in IR spectroscopy. (organic chemistry, analytical chemistry, IR spectroscopy)
What is the characteristic peak for nitriles in IR spectroscopy?
+
The characteristic peak for nitriles is the C≡N stretch, appearing between 2210–2260 cm-1.
How can I confirm the presence of a nitrile group in an IR spectrum?
+
Look for the sharp and intense C≡N stretch peak and compare it with reference spectra of known nitriles.
Why is baseline correction important in IR spectrum analysis?
+
Baseline correction ensures accurate peak positioning, preventing misinterpretation of spectral data.


