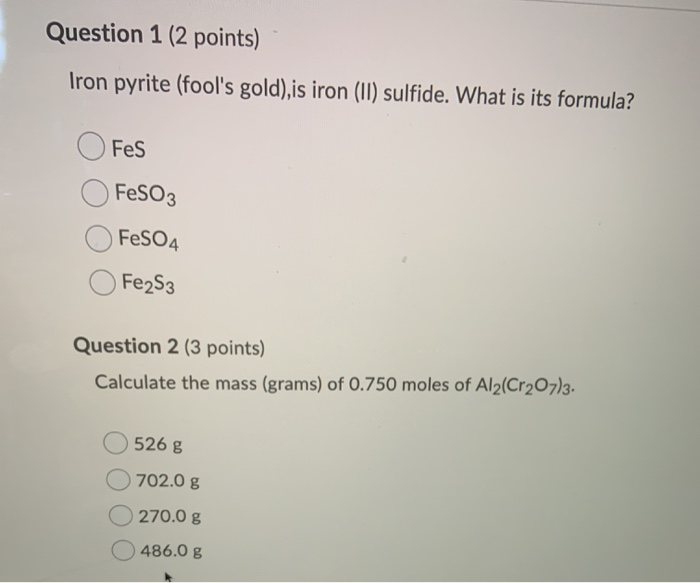
Iron Sulfide Formula: Quick Chemistry Guide

<!DOCTYPE html>
Iron sulfide, a compound formed by the reaction of iron and sulfur, is a topic of interest in both academic and industrial chemistry. Understanding its formula and properties is essential for students, researchers, and professionals alike. This guide provides a concise yet comprehensive overview of the iron sulfide formula, its derivation, and practical applications.
What is Iron Sulfide?
Iron sulfide, commonly known as iron(II) sulfide or ferrous sulfide, is a chemical compound with the formula FeS. It is a black, crystalline solid that occurs naturally as the mineral pyrite or “fool’s gold.” Iron sulfide is widely used in industries such as mining, metallurgy, and water treatment.
Deriving the Iron Sulfide Formula
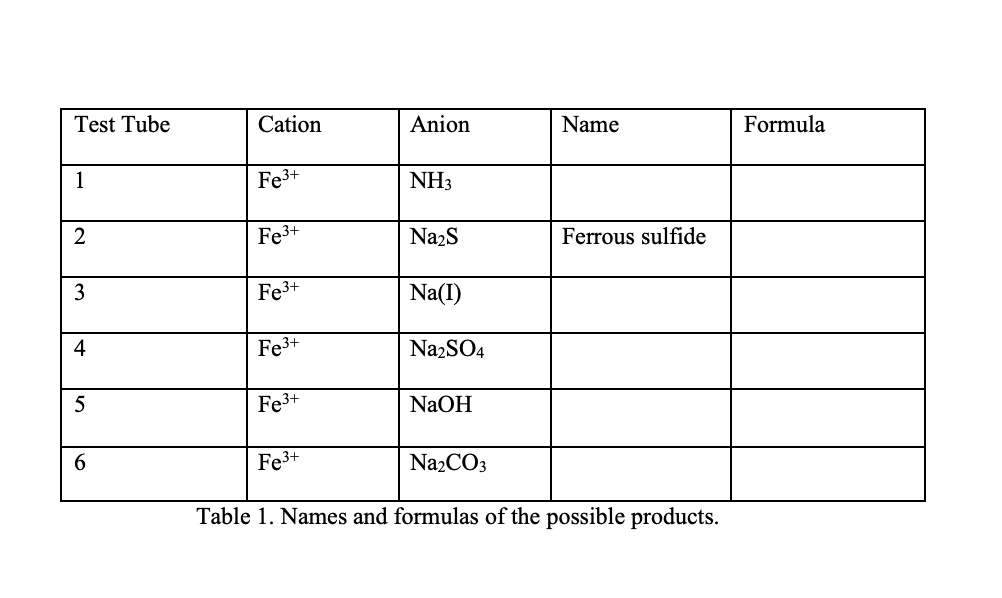
The formula for iron sulfide (FeS) is derived from the combination of iron (Fe) and sulfur (S) in a 1:1 ratio. Here’s a step-by-step breakdown:
- Iron (Fe): A transition metal with a +2 oxidation state in this compound.
- Sulfur (S): A non-metal with a -2 oxidation state.
When iron and sulfur react, they form FeS, balancing the charges and creating a stable compound. Chemical equation: Fe + S → FeS.
Properties of Iron Sulfide
Understanding the properties of iron sulfide is crucial for its practical applications. Below are key characteristics:
| Property | Description |
|---|---|
| Appearance | Black, crystalline solid |
| Melting Point | 1,188°C (2,170°F) |
| Solubility | Insoluble in water, soluble in acids |
| Uses | Mining, metallurgy, water treatment |
Practical Applications of Iron Sulfide

Iron sulfide has diverse applications across industries. Here are some notable uses:
- Mining: Extracted as pyrite for sulfur and iron production.
- Metallurgy: Used in the purification of metals.
- Water Treatment: Removes heavy metals from wastewater.
📌 Note: Iron sulfide is also used in the production of fertilizers and as a semiconductor material in certain electronic applications.
Safety and Handling

While iron sulfide is relatively stable, proper handling is essential to avoid risks:
- Wear protective gear when handling.
- Store in a cool, dry place away from oxidizing agents.
- Dispose of according to local regulations.
Understanding the iron sulfide formula (FeS) and its properties is fundamental in chemistry and industry. From its derivation to practical applications, this guide provides a quick yet detailed overview. Whether you're a student or a professional, mastering this compound enhances your knowledge and skills in the field. (iron sulfide uses, iron sulfide properties, chemical compounds)
What is the chemical formula for iron sulfide?
+The chemical formula for iron sulfide is FeS.
What are the main uses of iron sulfide?
+Iron sulfide is used in mining, metallurgy, water treatment, and as a semiconductor material.
Is iron sulfide soluble in water?
+No, iron sulfide is insoluble in water but soluble in acids.



