Is Helium Gas Diatomic? The Surprising Truth Revealed

Helium, the second most abundant element in the universe, is widely recognized for its use in balloons and its unique ability to make voices squeaky. But when it comes to its molecular structure, there’s often confusion: is helium gas diatomic? This question sparks curiosity among students, scientists, and enthusiasts alike. In this blog, we’ll dive into the surprising truth about helium’s molecular nature, exploring its properties, behavior, and why it stands out from other gases. Whether you’re here for academic knowledge or practical applications, this guide will clarify all your doubts about helium’s diatomic status,helium gas properties,helium molecular structure.
Understanding Diatomic Molecules: A Quick Primer

Before we address whether helium is diatomic, let’s understand what diatomic molecules are. Diatomic molecules consist of two atoms bonded together, typically of the same element. Examples include oxygen (O₂), hydrogen (H₂), and nitrogen (N₂). These molecules are common in nature and play crucial roles in various scientific and industrial processes,diatomic molecules definition,examples of diatomic molecules.
Is Helium Gas Diatomic? The Surprising Answer

Here’s the surprising truth: helium gas is not diatomic. Unlike oxygen or hydrogen, helium exists as single atoms in its gaseous state. This is because helium is a noble gas with a full outer electron shell, making it highly stable and unlikely to form bonds with other atoms,helium noble gas,helium single atoms.
💡 Note: Helium’s non-diatomic nature is due to its complete electron configuration, which eliminates the need to share or gain electrons.
Why Helium Doesn’t Form Diatomic Molecules
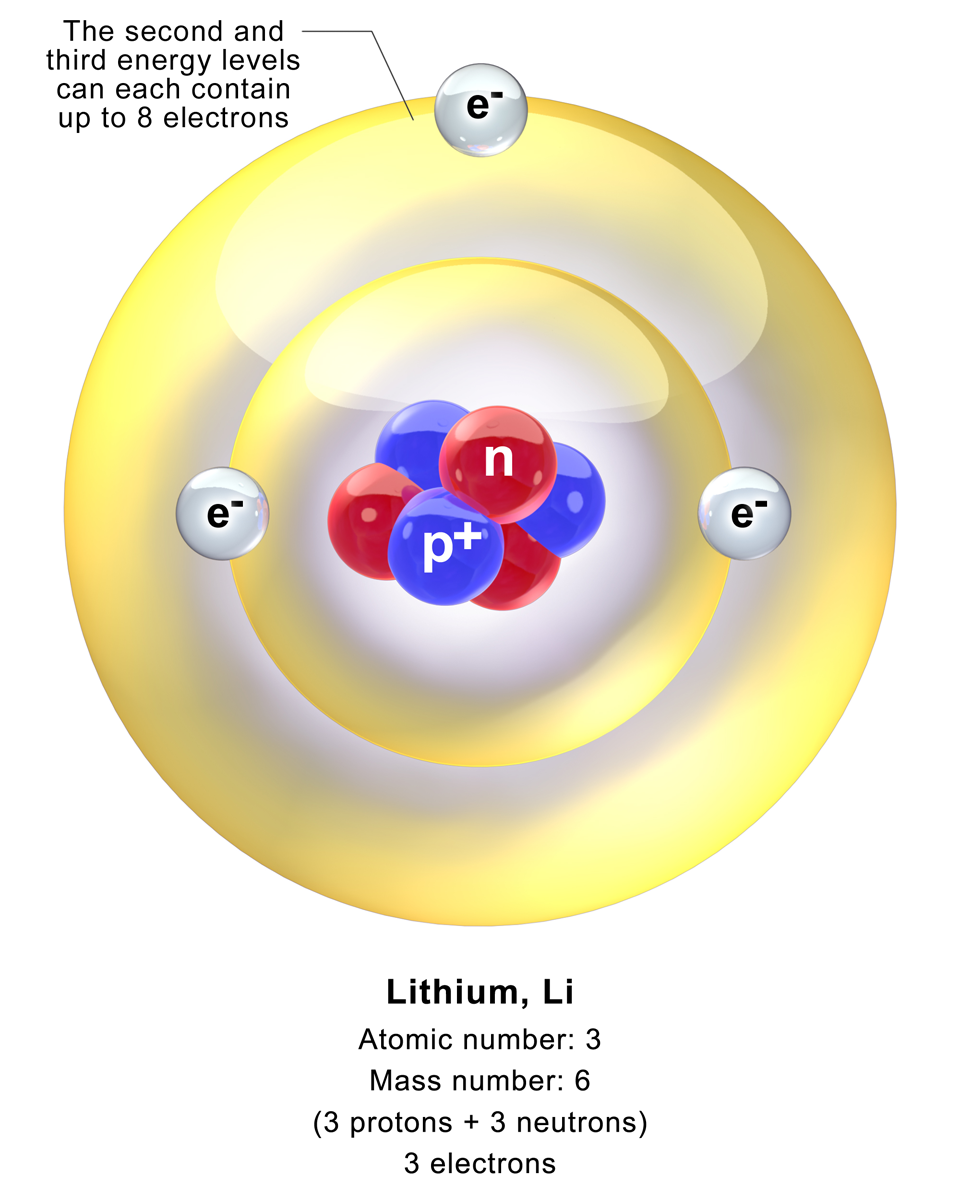
Helium’s unique properties stem from its position on the periodic table. As a Group 18 element, it has a full valence shell, making it chemically inert. This stability means helium atoms have no tendency to bond with each other, unlike elements in Groups 16 and 17, which often form diatomic molecules to achieve stability,periodic table groups,chemical stability of helium.
| Element | Molecular Form | Reason |
|---|---|---|
| Helium (He) | Single atoms | Full valence shell, chemically inert |
| Oxygen (O₂) | Diatomic | Needs to share electrons for stability |
| Hydrogen (H₂) | Diatomic | Needs to share electrons for stability |
Practical Implications of Helium’s Non-Diatomic Nature

Understanding helium’s molecular structure is crucial for its applications. Since helium atoms are monoatomic, they exhibit unique behaviors, such as:
- Low boiling and melting points due to weak interatomic forces.
- High thermal conductivity, making it ideal for cooling applications.
- Inertness, which ensures safety in environments like MRI machines and airships,helium applications,thermal conductivity of helium.
Key Takeaways: Helium’s Molecular Structure
- Helium gas is not diatomic; it exists as single atoms.
- Its non-diatomic nature is due to its full valence shell and chemical inertness.
- Helium’s monoatomic structure influences its unique properties and applications.
In summary, helium gas is not diatomic, setting it apart from many other gases. Its monoatomic nature, driven by its full electron configuration, explains its stability and unique properties. Whether you’re a student, scientist, or simply curious, understanding helium’s molecular structure sheds light on its importance in both science and everyday life,helium gas properties,helium molecular structure.
Why is helium not diatomic?
+
Helium is not diatomic because it has a full valence shell, making it chemically inert and unlikely to form bonds with other atoms,helium noble gas,chemical inertness.
What are diatomic molecules?
+
Diatomic molecules consist of two atoms bonded together, usually of the same element, such as O₂ or H₂,diatomic molecules definition,examples of diatomic molecules.
How does helium’s monoatomic nature affect its uses?
+
Helium’s monoatomic structure gives it low boiling and melting points, high thermal conductivity, and inertness, making it ideal for cooling and safe industrial applications,helium applications,thermal conductivity of helium.



