Is OF2 Polar or Nonpolar? Uncover the Truth.
Is OF2 Polar or Nonpolar? Unveiling the Molecular Truth
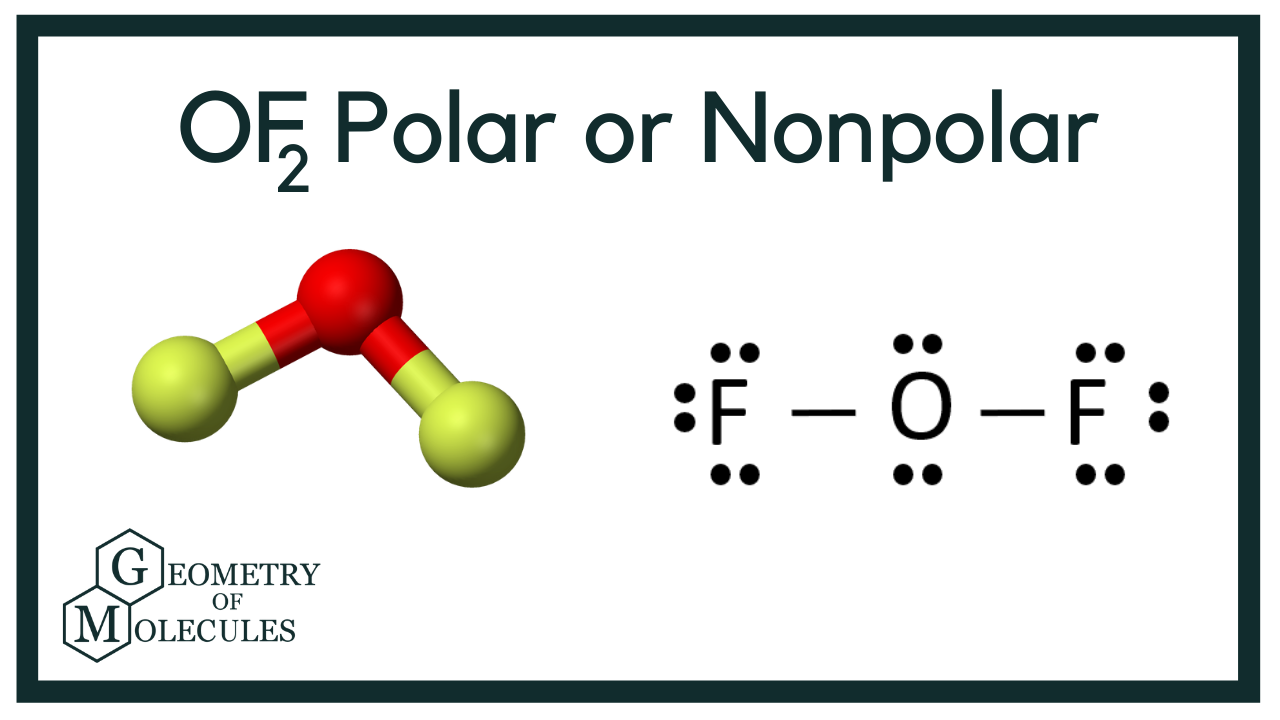
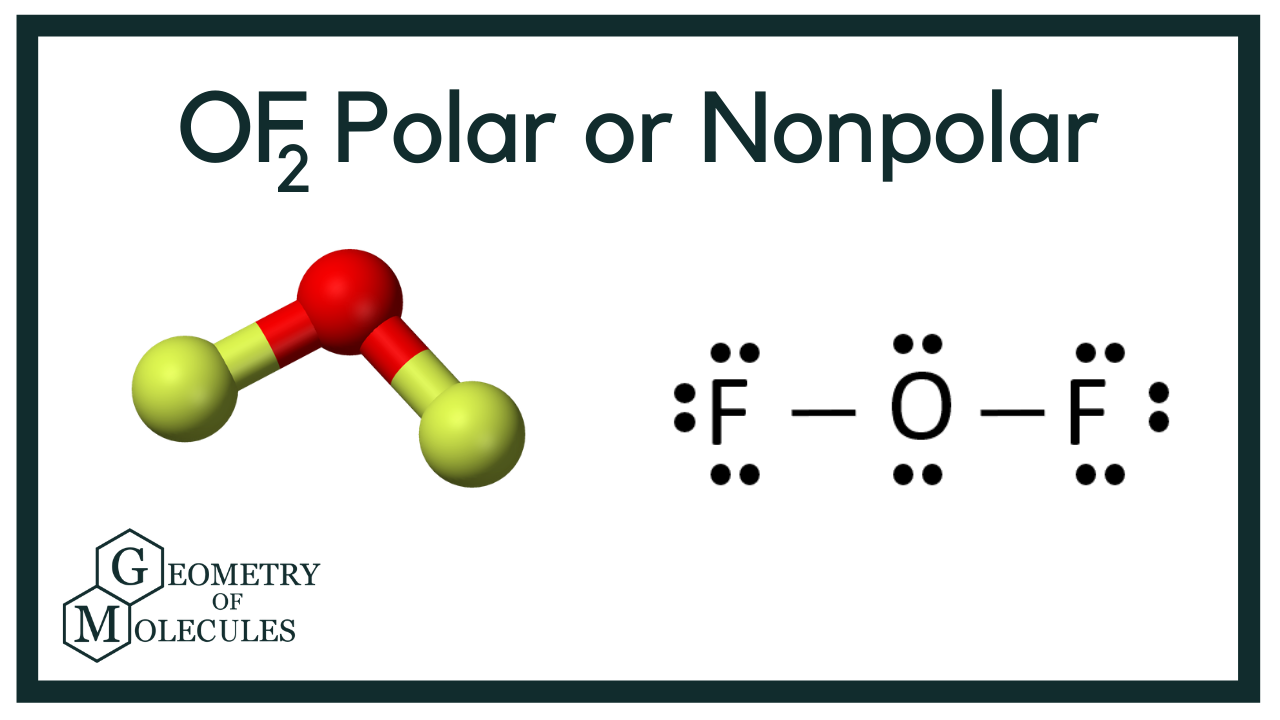
Oxygen difluoride (OF₂) is a molecule that often sparks curiosity due to its unique composition. With oxygen at its center and two fluorine atoms bonded to it, understanding its polarity is crucial for grasping its chemical behavior.
Understanding Polarity in Molecules
Polarity in a molecule arises from the uneven distribution of charge, leading to a dipole moment. This occurs when there’s a significant difference in electronegativity between the atoms involved in the bond.
- Electronegativity: A measure of an atom’s ability to attract electrons in a bond.
- Dipole Moment: A vector quantity representing the separation of charge in a bond.
OF₂: Analyzing the Molecular Structure
OF₂ has a bent molecular geometry due to the presence of two lone pairs on the oxygen atom. This geometry plays a key role in determining its polarity.
| Property | OF₂ |
|---|---|
| Molecular Geometry | Bent |
| Electronegativity Difference (O-F) | Fluorine is more electronegative than oxygen |
| Dipole Moment | Present due to bent shape |

Is OF₂ Polar or Nonpolar?
Despite the O-F bonds being polar due to fluorine’s higher electronegativity, OF₂ is polar overall because of its bent shape. The bond dipoles do not cancel out, resulting in a net dipole moment.
💡 Note: The bent geometry of OF₂ ensures that the individual bond dipoles do not cancel each other out, making the molecule polar.
Key Factors Determining OF₂’s Polarity
- Electronegativity Difference: Fluorine is more electronegative than oxygen.
- Molecular Geometry: Bent shape prevents dipole cancellation.
- Lone Pairs: Two lone pairs on oxygen contribute to the bent structure.
Checklist: Determining Polarity in Molecules
- Check Electronegativity: Identify the difference between bonded atoms.
- Analyze Geometry: Determine if the molecule is linear, bent, or trigonal.
- Assess Dipole Cancellation: See if bond dipoles cancel out.
(Molecular Polarity, Chemical Bonding, Electronegativity)
Final Thoughts

OF₂ is polar due to its bent molecular geometry and the presence of a net dipole moment. Understanding its polarity is essential for predicting its reactivity and physical properties. Whether you’re a student or a chemistry enthusiast, grasping these concepts enhances your knowledge of molecular interactions.
What makes OF₂ polar?
+
OF₂ is polar due to its bent molecular geometry and the presence of a net dipole moment caused by the electronegativity difference between oxygen and fluorine.
How does molecular geometry affect polarity?
+
Molecular geometry determines whether bond dipoles cancel out. In OF₂, the bent shape prevents cancellation, making it polar.
Why is electronegativity important in determining polarity?
+
Electronegativity differences between atoms create partial charges, leading to polar bonds. In OF₂, fluorine’s higher electronegativity makes the O-F bonds polar.



