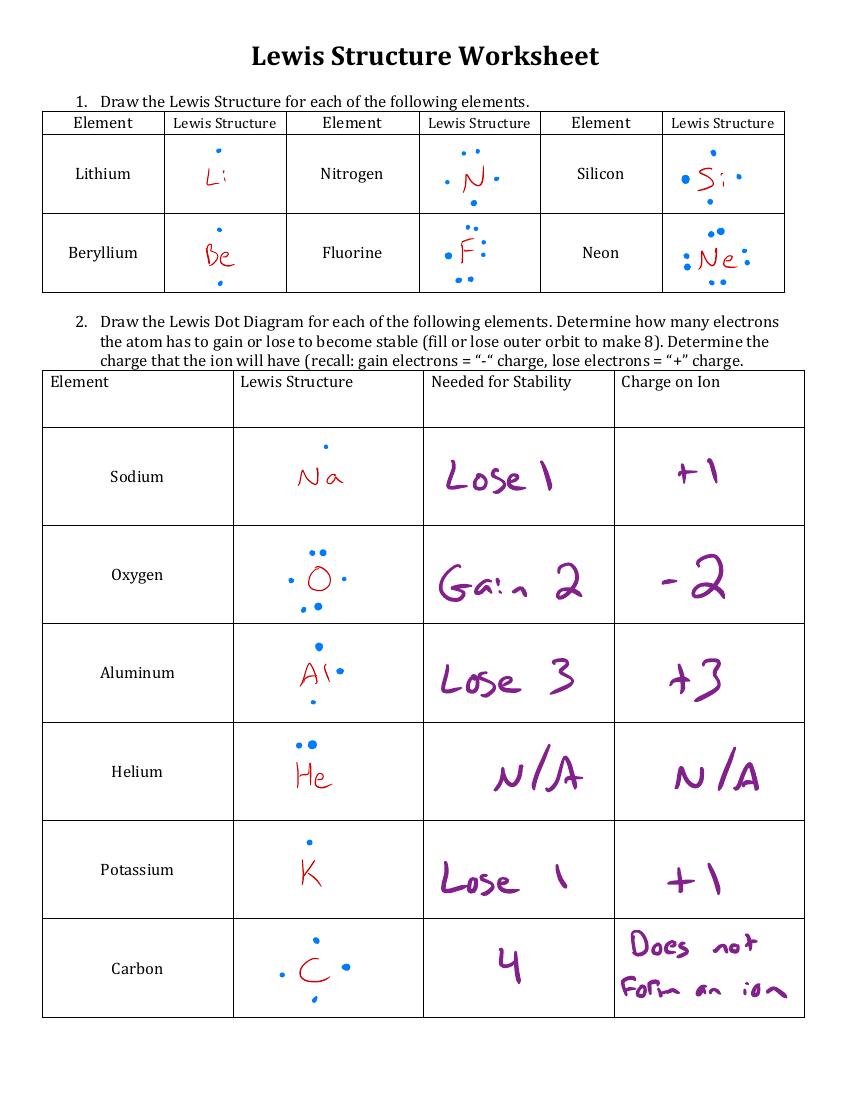
lewis dot practice worksheet

<!DOCTYPE html>
Mastering the Lewis Dot Structure is essential for understanding chemical bonding. Whether you’re a student preparing for exams or a chemistry enthusiast, practicing with a Lewis Dot Practice Worksheet can significantly enhance your skills. This guide provides step-by-step instructions, tips, and resources to help you excel in drawing Lewis Dot Structures.
What is a Lewis Dot Structure?

A Lewis Dot Structure, also known as an electron dot diagram, represents the distribution of valence electrons in an atom or molecule. It helps visualize how atoms bond and share electrons. Understanding this concept is crucial for predicting molecular geometry and reactivity, (lewis dot structure,chemical bonding,valence electrons).
How to Use a Lewis Dot Practice Worksheet
Step 1: Count Valence Electrons
Begin by determining the number of valence electrons for each atom in the molecule. Use the periodic table as a reference. For example, carbon has 4 valence electrons, while oxygen has 6, (periodic table,valence electrons,molecular structure).
Step 2: Draw the Skeleton Structure
Arrange the atoms with the least electronegative atom in the center. Connect the atoms with single bonds, ensuring the structure is stable and balanced, (electronegativity,molecular geometry,chemical bonds).
Step 3: Distribute Electrons
Place dots around each atom to represent valence electrons. Start by satisfying the octet rule for each atom, ensuring all atoms have a full outer shell, (octet rule,electron distribution,chemical bonding).
📌 Note: Hydrogen and helium follow the duet rule, requiring only 2 electrons to be stable.
Step 4: Check for Stability
Verify that the structure is stable by ensuring all atoms have a complete octet (or duet for hydrogen). If not, consider multiple bonds or resonance structures, (resonance structures,molecular stability,chemical reactions).
Benefits of Practicing with a Worksheet
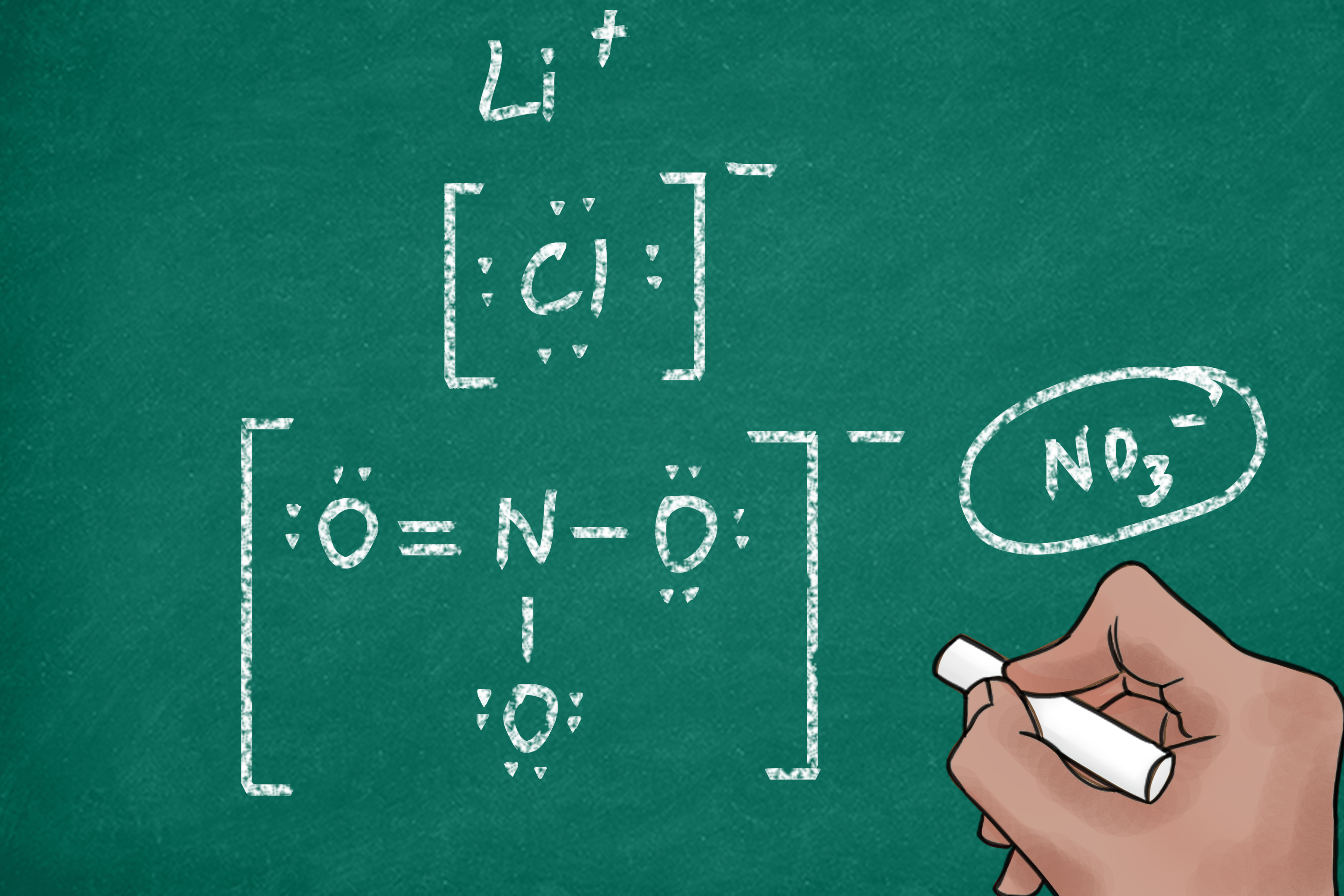
- Reinforces understanding of valence electrons and bonding.
- Improves problem-solving skills in chemistry.
- Prepares for exams and assessments.
- Enhances ability to predict molecular properties, (problem-solving,exam preparation,molecular properties).
Where to Find Lewis Dot Practice Worksheets

Numerous online resources offer free and paid worksheets. Look for reputable educational websites or chemistry-focused platforms. These worksheets often include answers for self-assessment, (online resources,educational tools,chemistry practice).
Checklist for Mastering Lewis Dot Structures

- Count valence electrons accurately.
- Draw the correct skeleton structure.
- Distribute electrons following the octet rule.
- Check for stability and adjust as needed.
- Practice regularly with diverse molecules, (practice tips,chemistry skills,molecular practice).
By following these steps and utilizing a Lewis Dot Practice Worksheet, you’ll build a strong foundation in chemical bonding. Consistent practice is key to mastering this essential chemistry skill, (chemistry mastery,chemical bonding,lewis dot practice).
What is the purpose of a Lewis Dot Structure?
+A Lewis Dot Structure helps visualize the distribution of valence electrons and how atoms bond in a molecule, (lewis dot structure,chemical bonding,valence electrons).
How do I count valence electrons?
+Use the periodic table to determine the number of valence electrons for each atom based on its group number, (periodic table,valence electrons,chemistry basics).
What is the octet rule?
+The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of 8 electrons, (octet rule,electron configuration,chemical stability).



