Chloroform Lewis Dot Structure: Quick Guide & Tips

Understanding the Chloroform Lewis Dot Structure is essential for students and professionals in chemistry. This structure helps visualize the electron distribution and bonding in chloroform (CHCl₃), a common organic solvent. Below, we’ll guide you through the process step-by-step, offering tips and insights to master this concept.
What is the Chloroform Lewis Dot Structure?
The Lewis Dot Structure represents the arrangement of atoms and valence electrons in a molecule. For chloroform (CHCl₃), it shows how carbon ©, hydrogen (H), and chlorine (Cl) atoms bond and share electrons. This structure is crucial for understanding molecular geometry and reactivity.
Step-by-Step Guide to Drawing the Chloroform Lewis Dot Structure
- Determine the Central Atom: Carbon © is the central atom in chloroform.
- Count Valence Electrons:
- Carbon: 4 electrons
- Hydrogen: 1 electron
- Chlorine: 7 electrons (×3)
Total: 4 + 1 + (7 × 3) = 26 electrons.
- Carbon: 4 electrons
- Arrange Atoms: Place carbon in the center, surrounded by hydrogen and chlorine atoms.
- Form Bonds: Create single bonds between carbon and each atom (H, Cl₃).
- Complete Octets: Distribute remaining electrons to satisfy the octet rule for chlorine atoms.
✨ Note: Hydrogen only needs 2 electrons to complete its shell, while chlorine requires 8.
Tips for Mastering Lewis Dot Structures
- Start with the Least Electronegative Atom: Carbon is the central atom due to its lower electronegativity compared to chlorine.
- Use Double or Triple Bonds if Necessary: Chloroform only requires single bonds, but this tip is useful for other molecules.
- Check Formal Charges: Ensure the structure has the lowest possible formal charges for stability.
Chloroform Lewis Dot Structure: Key Takeaways
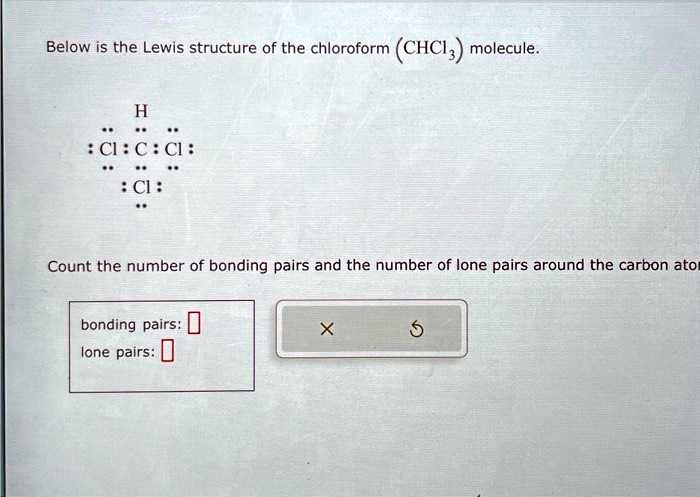
- Carbon forms single bonds with hydrogen and three chlorine atoms.
- The structure has 26 valence electrons, with chlorine atoms completing their octets.
- Understanding this structure aids in predicting chloroform’s chemical behavior.
Checklist for Drawing Lewis Dot Structures
- [ ] Identify the central atom.
- [ ] Count total valence electrons.
- [ ] Arrange atoms and form bonds.
- [ ] Distribute remaining electrons to complete octets.
- [ ] Verify formal charges for stability.
Chloroform Lewis Dot Structure,Lewis Dot Structure,Chloroform Molecular Structure
What is the central atom in chloroform?
+Carbon (C) is the central atom in chloroform (CHCl₃).
How many valence electrons does chloroform have?
+Chloroform has 26 valence electrons (4 from C, 1 from H, and 21 from Cl₃).
Why is the Lewis Dot Structure important?
+It helps visualize molecular bonding and electron distribution, aiding in understanding chemical properties.
In summary, mastering the Chloroform Lewis Dot Structure is a fundamental skill in chemistry. By following the steps and tips outlined above, you can confidently draw and analyze this structure. Remember to focus on electron distribution and bonding to predict molecular behavior accurately. Whether you’re a student or a professional, this guide ensures you have the tools to succeed.
Chloroform Lewis Dot Structure,Lewis Dot Structure,Chloroform Molecular Structure


