lewis dot structure of scl4

Understanding the Lewis Dot Structure of SCl4 (Sulfur Tetrafluoride) is essential for grasping its molecular geometry and chemical properties. This compound, composed of one sulfur atom and four fluorine atoms, is a fascinating subject in chemistry due to its unique structure and reactivity. Whether you’re a student, researcher, or simply curious about molecular structures, this guide will walk you through the Lewis dot structure of SCl4, its significance, and practical applications.
What is the Lewis Dot Structure?
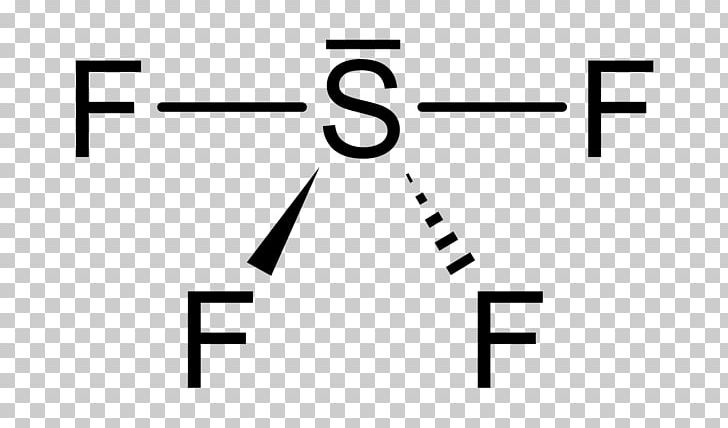
The Lewis dot structure is a diagram that represents the distribution of valence electrons in a molecule. It helps predict molecular shape, polarity, and bonding patterns. For SCl4, understanding its Lewis structure is crucial to analyzing its properties, such as its tetrahedral geometry and polar nature.
Steps to Draw the Lewis Dot Structure of SCl4
Determine the Total Valence Electrons:
- Sulfur (S) has 6 valence electrons.
- Each Fluorine (F) atom has 7 valence electrons.
- Total: (6 + 4 \times 7 = 34) valence electrons.
- Sulfur (S) has 6 valence electrons.
Choose the Central Atom:
- Sulfur (S) is the central atom due to its lower electronegativity compared to fluorine.
- Sulfur (S) is the central atom due to its lower electronegativity compared to fluorine.
Connect the Atoms:
- Draw single bonds between sulfur and each fluorine atom, using 8 electrons (4 bonds).
- Draw single bonds between sulfur and each fluorine atom, using 8 electrons (4 bonds).
Complete the Octets:
- Place the remaining 26 electrons as lone pairs on the fluorine atoms to satisfy their octets.
- Place the remaining 26 electrons as lone pairs on the fluorine atoms to satisfy their octets.
Check Formal Charges:
- Ensure the formal charges are minimized. In SCl4, the structure is stable with no additional charges.
- Ensure the formal charges are minimized. In SCl4, the structure is stable with no additional charges.
📌 Note: Sulfur expands its octet in SCl4, allowing it to form four bonds with fluorine atoms.
Molecular Geometry of SCl4
The molecular geometry of SCl4 is tetrahedral, with bond angles of approximately 102 degrees. This geometry arises from the four bonding pairs of electrons around the sulfur atom. The presence of lone pairs on fluorine atoms does not affect the overall shape but influences the molecule’s polarity.
Polarity of SCl4
SCl4 is a polar molecule due to the electronegativity difference between sulfur and fluorine. The fluorine atoms pull electron density away from sulfur, creating a net dipole moment.
Practical Applications of SCl4

SCl4 is used in various industries, including:
- Chemical Synthesis: As a fluorinating agent.
- Research: To study molecular interactions and bonding theories.
- Material Science: In the production of specialized materials.
Checklist for Drawing Lewis Dot Structures
- Count total valence electrons.
- Identify the central atom.
- Form single bonds between atoms.
- Complete octets with lone pairs.
- Verify formal charges for stability.
Wrapping Up

The Lewis dot structure of SCl4 provides valuable insights into its molecular geometry, polarity, and applications. By following the steps outlined above, you can confidently draw the structure and understand its chemical behavior. Whether for academic purposes or practical applications, mastering this concept is a significant step in your chemistry journey.
What is the molecular geometry of SCl4?
+The molecular geometry of SCl4 is tetrahedral, with bond angles of approximately 102 degrees.
Is SCl4 a polar or nonpolar molecule?
+SCl4 is a polar molecule due to the electronegativity difference between sulfur and fluorine atoms.
How many valence electrons are in SCl4?
+SCl4 has a total of 34 valence electrons (6 from sulfur and 7 from each of the four fluorine atoms).
Related Keywords: Lewis structure of SCl4, SCl4 molecular geometry, SCl4 polarity, sulfur tetrafluoride structure, how to draw SCl4 Lewis dot structure.



