lewis structure neon: A Clear and Concise Guide

Understanding the Lewis structure of neon (Ne) is essential for grasping its chemical behavior and properties. Neon, a noble gas, is unique due to its stable electron configuration, which makes it unreactive under normal conditions. This guide provides a clear and concise explanation of neon Lewis structure, its significance, and how to draw it accurately.
What is the Lewis Structure of Neon?

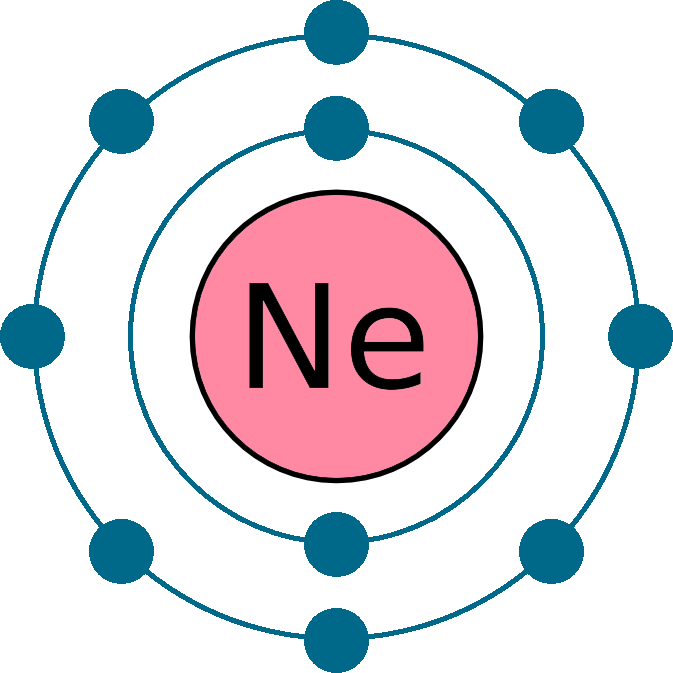
The Lewis structure represents the arrangement of electrons around atoms in a molecule or ion. For neon (Ne), the Lewis structure is straightforward due to its noble gas status. Neon has an atomic number of 10, meaning it has 10 electrons. In its ground state, neon’s electron configuration is 1s² 2s² 2p⁶, which corresponds to a full outer shell (octet).
💡 Note: Neon’s full outer shell makes it highly stable and unreactive, a hallmark of noble gases.
How to Draw the Lewis Structure of Neon
Drawing the Lewis structure of neon involves the following steps:
Determine the Total Number of Electrons:
Neon has 10 electrons (atomic number 10).Arrange Electrons Around the Atom:
Place dots around the symbol Ne to represent the electrons. Since neon has 8 valence electrons, they are arranged in pairs around the atom.Verify the Octet Rule:
Neon naturally satisfies the octet rule with 8 electrons in its outer shell.
The final Lewis structure of neon is represented as: Ne with 8 dots (4 pairs) around it.
| Step | Description |
|---|---|
| 1 | Count total electrons (10 for Ne) |
| 2 | Arrange electrons in pairs around Ne |
| 3 | Confirm the octet rule is satisfied |

Why is Neon’s Lewis Structure Important?

Neon’s Lewis structure highlights its stability and lack of reactivity. This is crucial in chemistry for understanding why noble gases rarely form compounds. Its full outer shell explains its inert nature, making it a benchmark for comparing other elements’ reactivity.
Key Takeaways
- Neon’s Lewis structure is simple due to its full outer shell (octet).
- It has 10 electrons, with 8 valence electrons arranged in pairs.
- The structure confirms neon’s stability and non-reactivity.
What is the Lewis structure of neon?
+The Lewis structure of neon (Ne) consists of the symbol "Ne" surrounded by 8 dots (4 pairs), representing its 8 valence electrons.
Why is neon unreactive?
+Neon is unreactive because it has a full outer shell (octet) of electrons, making it highly stable and unlikely to form bonds.
How does neon satisfy the octet rule?
+Neon naturally satisfies the octet rule with 8 valence electrons in its outermost shell, requiring no additional electrons to stabilize.
In summary, the Lewis structure of neon is a fundamental concept that illustrates its stability and inertness. By mastering this structure, you gain insights into the behavior of noble gases and their role in chemistry. Whether for academic purposes or practical applications, understanding neon’s Lewis structure is a valuable skill. (lewis structure of neon, noble gas electron configuration, octet rule in chemistry)



