lewis symbol for potassium explained simply

Understanding the Lewis symbol for potassium is essential for anyone studying chemistry, especially when it comes to grasping electron configurations and chemical bonding. Potassium, with its atomic number 19, is a highly reactive alkali metal that readily loses its outermost electron to achieve a stable state. The Lewis symbol simplifies this concept by visually representing potassium’s valence electrons. In this post, we’ll break down the Lewis symbol for potassium in simple terms, explain its significance, and provide practical insights for both informational and commercial purposes. Whether you’re a student, educator, or industry professional, this guide will help you master the topic.
What is a Lewis Symbol? (lewis dot structure,electron configuration)
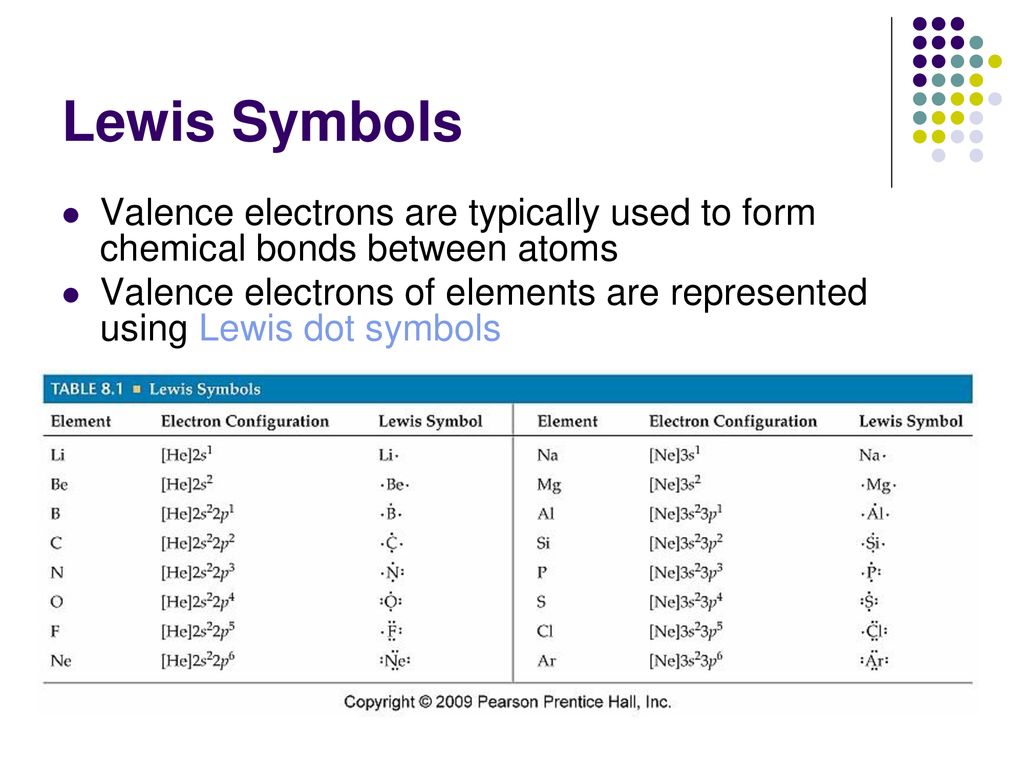
A Lewis symbol, also known as a Lewis dot structure, is a diagram that represents the valence electrons of an atom. Valence electrons are the electrons in the outermost shell of an atom, which determine its chemical properties. Lewis symbols use dots around the chemical symbol to indicate these electrons.
For example, the Lewis symbol for potassium (K) focuses on its single valence electron. Since potassium has an electron configuration of 2, 8, 8, 1, the lone electron in the fourth shell is represented in its Lewis symbol.
Lewis Symbol for Potassium Explained (potassium electron configuration,alkali metals)

The Lewis symbol for potassium is straightforward. Here’s how it’s represented:
- The chemical symbol K is written.
- A single dot is placed on one side of the symbol to represent its valence electron.
Visually, it looks like this: K·. This simple representation highlights potassium’s tendency to lose its outermost electron, which is a key characteristic of alkali metals.
Why is the Lewis Symbol Important? (chemical bonding,periodic table)
The Lewis symbol is crucial for understanding chemical bonding and predicting how atoms will interact. For potassium, the Lewis symbol shows its eagerness to form a +1 ion by losing its valence electron. This property is vital in:
- Forming ionic compounds, such as potassium chloride (KCl).
- Understanding its role in the periodic table as an alkali metal.
- Explaining its high reactivity with water and other substances.
📌 Note: The Lewis symbol for potassium is a simplified representation and does not account for its full electron configuration. It focuses solely on the valence electron.
Practical Applications of Potassium (potassium compounds,industrial uses)
For those with commercial interests, potassium plays a significant role in various industries. Its reactivity, as depicted in its Lewis symbol, makes it valuable in:
- Producing potassium compounds like fertilizers (e.g., potassium nitrate).
- Manufacturing soaps, detergents, and glass.
- Use in batteries and as a heat exchange medium in nuclear reactors.
Understanding potassium’s Lewis symbol helps industries optimize its use in chemical processes.
Checklist: Key Takeaways (lewis symbol tutorial,chemistry basics)

- Potassium’s Lewis symbol is K·, representing its single valence electron.
- It highlights potassium’s tendency to form a +1 ion.
- The symbol is essential for understanding chemical bonding and reactivity.
- Potassium’s properties make it valuable in agriculture, manufacturing, and energy sectors.
In summary, the Lewis symbol for potassium is a simple yet powerful tool for understanding its chemical behavior. Whether you’re studying chemistry basics or exploring industrial applications, mastering this concept will deepen your knowledge of potassium’s role in the periodic table and its practical uses. By focusing on its single valence electron, you can predict its reactivity and importance in various fields.
What is the Lewis symbol for potassium?
+
The Lewis symbol for potassium is K·, representing its single valence electron.
Why does potassium have only one dot in its Lewis symbol?
+
Potassium has one dot because it has only one valence electron in its outermost shell, as shown in its electron configuration 2, 8, 8, 1.
How does the Lewis symbol relate to potassium’s reactivity?
+
The single dot in potassium’s Lewis symbol indicates its willingness to lose that electron, making it highly reactive, especially with elements seeking electrons.
What are common uses of potassium in industry?
+
Potassium is widely used in fertilizers, soaps, detergents, glass manufacturing, and as a heat exchange medium in nuclear reactors.



