Molar Mass of Potassium: Quick Facts & Calculations
Understanding the molar mass of potassium is essential for students, chemists, and anyone working with this versatile element. Potassium, symbolized as K on the periodic table, plays a crucial role in various scientific and industrial applications. Whether you’re calculating chemical reactions or studying its properties, knowing its molar mass is fundamental. In this post, we’ll explore quick facts, calculations, and practical applications of potassium’s molar mass, tailored for both informational and commercial audiences.
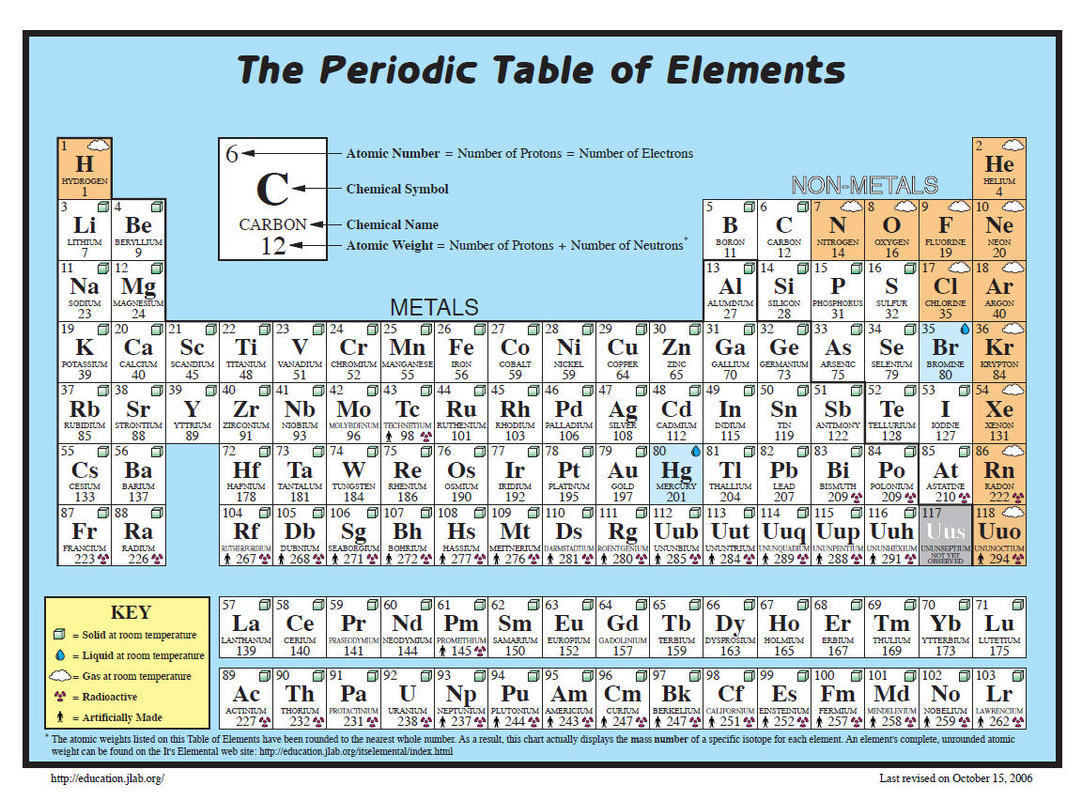
What is the Molar Mass of Potassium?

The molar mass of potassium is approximately 39.10 g/mol. This value represents the mass of one mole of potassium atoms. It’s derived from the atomic weight of potassium, which is based on its isotopes and their natural abundance. Understanding this value is key for stoichiometry, solution preparation, and other chemical calculations.
📌 Note: The molar mass is often rounded to 39.10 g/mol for simplicity in calculations.
How to Calculate the Molar Mass of Potassium

Calculating the molar mass of potassium is straightforward. Follow these steps:
- Identify the Atomic Mass: Potassium’s atomic mass is 39.0983 u (unified atomic mass units).
- Convert to Grams per Mole: Since 1 mole of any substance contains Avogadro’s number of particles, the atomic mass in grams gives the molar mass.
Thus, the molar mass of potassium is 39.10 g/mol.
Practical Applications of Potassium’s Molar Mass

The molar mass of potassium is vital in:
- Chemical Reactions: Balancing equations and determining reactant/product quantities.
- Pharmaceuticals: Formulating potassium-based medications.
- Agriculture: Producing potassium-rich fertilizers.
For commercial purposes, knowing the molar mass ensures accurate product formulation and quality control.
Quick Facts About Potassium

- Symbol: K
- Atomic Number: 19
- State at Room Temperature: Solid
- Common Uses: Fertilizers, batteries, and medical treatments.
| Property | Value |
|---|---|
| Molar Mass | 39.10 g/mol |
| Melting Point | 63.5°C |
| Boiling Point | 759°C |

Checklist for Molar Mass Calculations

- Verify the atomic mass of potassium.
- Use the correct units (g/mol).
- Double-check calculations for accuracy.
Understanding the molar mass of potassium is a foundational skill in chemistry. Whether for academic studies or industrial applications, this knowledge ensures precision in your work.
What is the molar mass of potassium?
+The molar mass of potassium is approximately 39.10 g/mol.
Why is the molar mass of potassium important?
+It’s crucial for chemical calculations, pharmaceutical formulations, and agricultural applications.
How do you calculate the molar mass of potassium?
+Use potassium’s atomic mass (39.0983 u) and convert it to grams per mole.
potassium properties,chemical calculations,atomic mass,periodic table,stoichiometry,