CH2O Molecular Geometry: Shape and Bond Angles Explained

Understanding the molecular geometry of CH2O (formaldehyde) is crucial for grasping its chemical properties and behavior. This blog post delves into the CH2O molecular geometry, its shape, bond angles, and the factors influencing its structure. Whether you’re a student, researcher, or simply curious about chemistry, this guide provides clear, SEO-optimized insights tailored to both informational and commercial audiences.
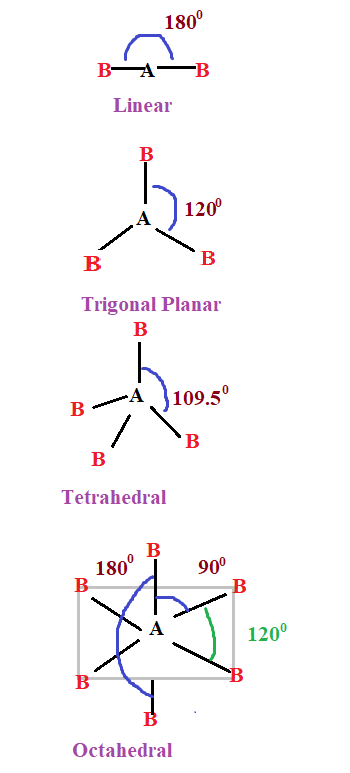
What is CH2O Molecular Geometry?

CH2O, or formaldehyde, is a simple organic compound composed of one carbon atom, two hydrogen atoms, and one oxygen atom. Its molecular geometry is determined by the arrangement of these atoms in space. The shape of CH2O is trigonal planar, a common geometry for molecules with a central atom bonded to three other atoms and no lone pairs.
📌 Note: The trigonal planar shape is characterized by 120-degree bond angles between the atoms, ensuring maximum stability.
Bond Angles in CH2O

The bond angles in CH2O are approximately 120 degrees, a direct result of its trigonal planar geometry. This angle minimizes electron repulsion between the bonding pairs, contributing to the molecule’s stability. The double bond between carbon and oxygen does not alter the overall shape but affects the molecule’s reactivity.
Factors Influencing CH2O Geometry

Several factors determine the molecular geometry of CH2O:
- Electron Pair Repulsion: The VSEPR (Valence Shell Electron Pair Repulsion) theory explains how electron pairs around the central atom repel each other, leading to the trigonal planar shape.
- Hybridization: The carbon atom in CH2O undergoes sp2 hybridization, which aligns with the trigonal planar geometry.
- Formal Charge: The distribution of electrons ensures that the formal charge on each atom is minimized, stabilizing the molecule.
| Factor | Impact on CH2O Geometry |
|---|---|
| Electron Pair Repulsion | Determines 120-degree bond angles |
| Hybridization | Supports trigonal planar shape |
| Formal Charge | Ensures stability |
Practical Applications of CH2O Geometry

Understanding CH2O’s geometry is essential for various applications:
- Chemical Synthesis: Knowledge of its shape aids in designing reactions involving formaldehyde.
- Biological Systems: CH2O plays a role in metabolic processes, and its geometry influences its interactions with enzymes.
- Industrial Uses: Formaldehyde is used in resins, plastics, and preservatives, where its molecular structure is critical.
Key Takeaways

- CH2O molecular geometry is trigonal planar with 120-degree bond angles.
- sp2 hybridization and VSEPR theory explain its shape.
- Its geometry is vital for chemical, biological, and industrial applications.
What is the shape of CH2O?
+The shape of CH2O is trigonal planar due to its sp2 hybridization and the arrangement of atoms around the central carbon.
Why are the bond angles in CH2O 120 degrees?
+The 120-degree bond angles in CH2O result from the trigonal planar geometry, which minimizes electron pair repulsion.
How does CH2O geometry impact its reactivity?
+The trigonal planar shape and double bond in CH2O influence its reactivity, making it a versatile compound in chemical reactions.
In summary, the CH2O molecular geometry is a fundamental concept in chemistry, offering insights into its structure, stability, and applications. By understanding its trigonal planar shape and 120-degree bond angles, you can better appreciate its role in various scientific and industrial contexts. Whether for academic study or practical use, this knowledge is invaluable.
Related: molecular geometry, VSEPR theory, sp2 hybridization, formaldehyde uses, chemical bonding



