Molecular Mass of Sodium: Quick Facts & Calculation Guide
Understanding the molecular mass of sodium is essential in chemistry, whether you’re a student, researcher, or simply curious about the elements. Sodium, represented by the symbol Na, is a key element in various scientific and industrial applications. This guide provides quick facts and a step-by-step calculation method to determine its molecular mass.
What is the Molecular Mass of Sodium?

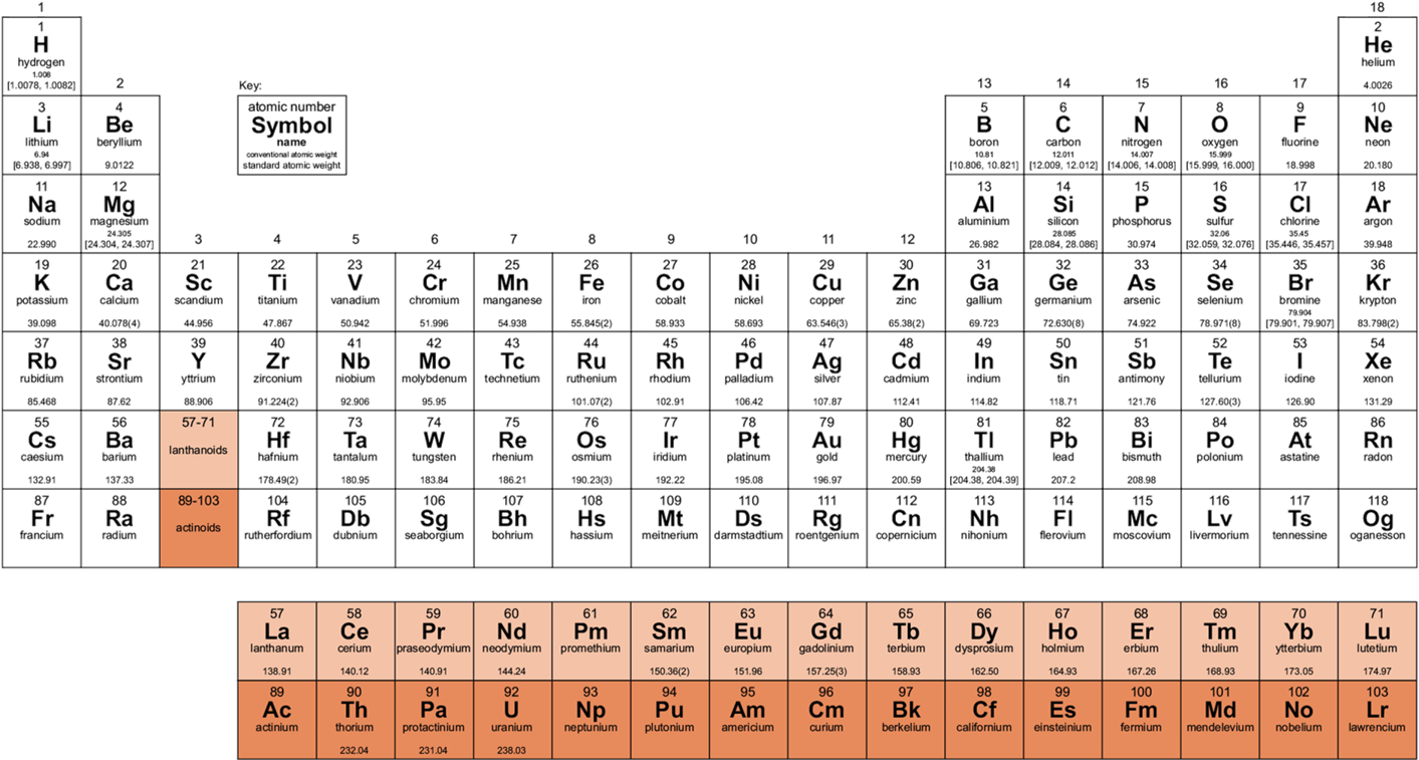
The molecular mass of sodium refers to the sum of the masses of its constituent atoms. Sodium is a single-atom element, so its molecular mass is equivalent to its atomic mass. The atomic mass of sodium is approximately 22.99 u (unified atomic mass units). This value is crucial for calculations in stoichiometry, chemical reactions, and more.
Quick Facts About Sodium
- Atomic Number: 11
- Symbol: Na
- Atomic Mass: 22.99 u
- State at Room Temperature: Solid
- Common Uses: Table salt (NaCl), soap, and industrial applications
| Property | Value |
|---|---|
| Atomic Number | 11 |
| Atomic Mass | 22.99 u |
| State at Room Temperature | Solid |

How to Calculate the Molecular Mass of Sodium

Calculating the molecular mass of sodium is straightforward since it’s a single-atom element. Follow these steps:
- Identify the Atomic Mass: Refer to the periodic table to find sodium’s atomic mass (22.99 u).
- Confirm the Element’s Purity: Ensure you’re working with pure sodium (Na).
- State the Result: The molecular mass of sodium is 22.99 u.
📌 Note: For compounds containing sodium, calculate the total molecular mass by summing the atomic masses of all constituent atoms.
Why is Sodium’s Molecular Mass Important?

Understanding sodium’s molecular mass is vital for:
- Chemical Reactions: Balancing equations accurately.
- Stoichiometry: Determining reactant and product quantities.
- Industrial Applications: Producing sodium-based compounds like sodium hydroxide (NaOH).
Key Takeaways

- Sodium’s molecular mass is 22.99 u.
- It’s a single-atom element, so its atomic and molecular masses are the same.
- This value is essential for chemical calculations and industrial processes.
What is the molecular mass of sodium?
+The molecular mass of sodium is 22.99 u.
How do you calculate sodium's molecular mass?
+Since sodium is a single-atom element, its molecular mass is equal to its atomic mass (22.99 u).
Why is sodium’s molecular mass important?
+It’s crucial for balancing chemical equations, stoichiometry, and industrial applications.
In summary, the molecular mass of sodium is a fundamental concept in chemistry, with its value (22.99 u) serving as a cornerstone for various scientific and industrial processes. Whether you’re studying chemistry or working in a lab, mastering this concept is essential.
(molecular mass of sodium, atomic mass of sodium, sodium properties, sodium in chemistry)



