NH3 Lewis Dot Structure: Simple Guide to Ammonia
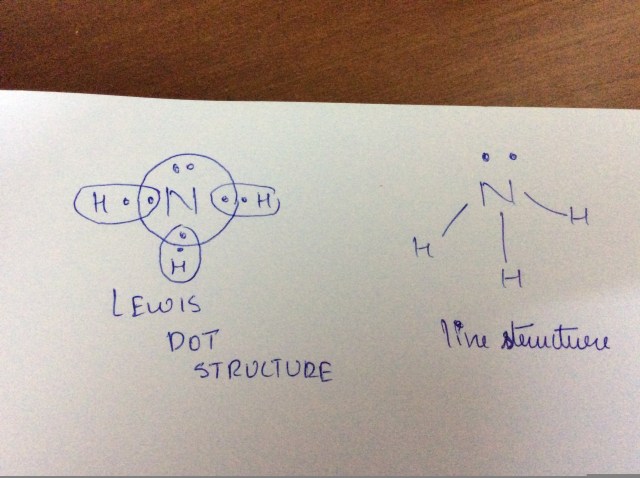
Understanding the NH3 Lewis dot structure is essential for anyone studying chemistry, especially when it comes to grasping the molecular geometry of ammonia (NH3). This simple guide will walk you through the step-by-step process of drawing the Lewis structure, explaining the electron arrangement, and highlighting the importance of this compound in various applications. Whether you're a student, a researcher, or simply curious, this post will provide clear insights into the molecular structure of ammonia, its bonding, and its significance in chemistry, (NH3 Lewis Structure, Ammonia Molecular Geometry, Chemical Bonding).
What is NH3 Lewis Dot Structure?

The NH3 Lewis dot structure represents the arrangement of atoms and electrons in an ammonia molecule. It consists of one nitrogen (N) atom bonded to three hydrogen (H) atoms, with a lone pair of electrons on the nitrogen atom. This structure helps visualize the electron distribution and understand the molecule’s geometry, which is crucial for predicting its chemical behavior, (Lewis Structure, Electron Distribution, Molecular Geometry).
Steps to Draw NH3 Lewis Structure

Drawing the NH3 Lewis structure is straightforward if you follow these steps:
- Step 1: Count the total valence electrons. Nitrogen has 5 valence electrons, and each hydrogen has 1, totaling 8 electrons.
- Step 2: Place atoms and form bonds. Position nitrogen in the center and connect it to the three hydrogen atoms using single bonds.
- Step 3: Complete the octet. Ensure each hydrogen has 2 electrons (a full outer shell), and place the remaining electrons as a lone pair on nitrogen.
💡 Note: Hydrogen atoms can only have 2 electrons, so they never form double or triple bonds, (Valence Electrons, Single Bonds, Octet Rule).
Molecular Geometry of NH3
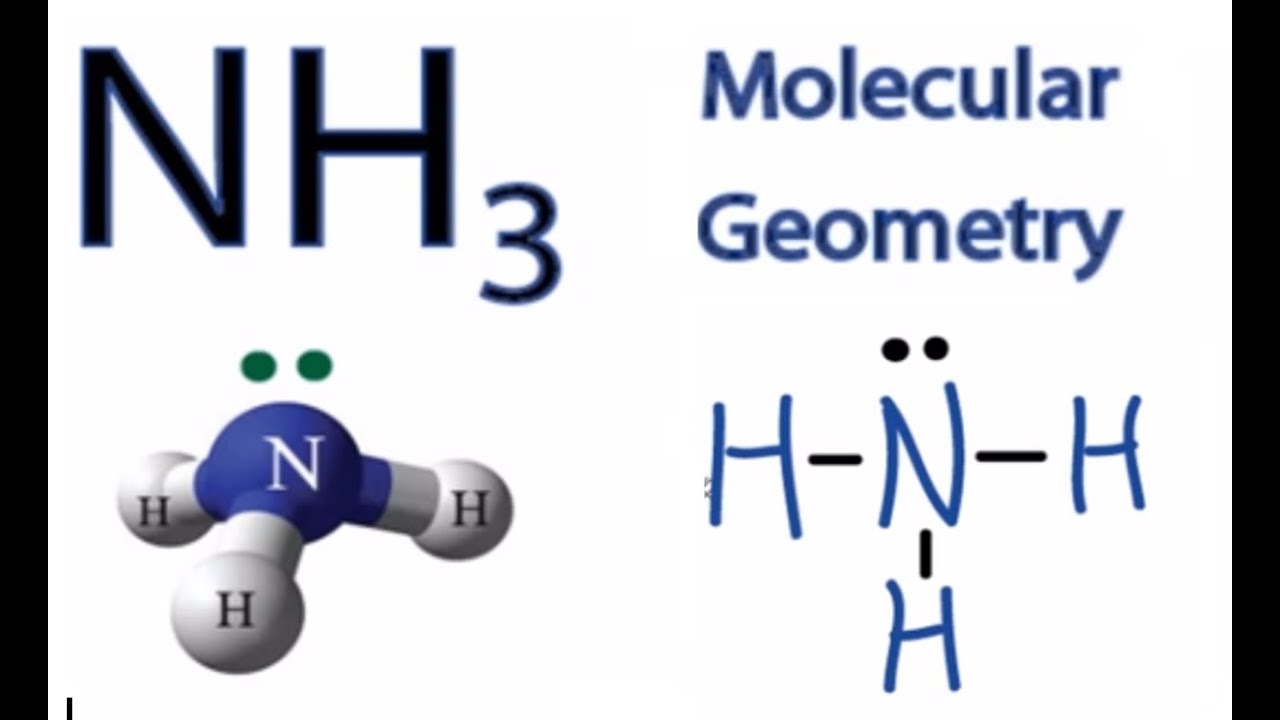
The molecular geometry of NH3 is trigonal pyramidal. This shape arises because the lone pair of electrons on the nitrogen atom repels the bonded pairs, causing the hydrogen atoms to move slightly apart. The bond angle in NH3 is approximately 107 degrees, which is less than the ideal 109.5 degrees due to lone pair repulsion, (Trigonal Pyramidal, Bond Angle, Lone Pair Repulsion).
| Molecule | Geometry | Bond Angle |
|---|---|---|
| NH3 | Trigonal Pyramidal | 107° |
| CH4 | Tetrahedral | 109.5° |

Applications of Ammonia
Ammonia (NH3) is a versatile compound with numerous applications, including:
- Fertilizers: NH3 is a key component in producing nitrogen-based fertilizers.
- Refrigeration: It is used as a refrigerant due to its excellent heat absorption properties.
- Cleaning Agents: Ammonia-based solutions are common in household cleaning products.
These applications highlight the importance of understanding the NH3 Lewis structure and its chemical properties, (Fertilizers, Refrigeration, Cleaning Agents).
In summary, the NH3 Lewis dot structure is a fundamental concept in chemistry that helps explain the molecular geometry and properties of ammonia. By following the steps outlined above, you can easily draw the structure and understand its significance in various fields. Whether for academic purposes or practical applications, mastering this concept is invaluable, (NH3 Lewis Structure, Ammonia Properties, Chemical Applications).
What is the molecular geometry of NH3?
+
The molecular geometry of NH3 is trigonal pyramidal due to the lone pair of electrons on the nitrogen atom.
How many valence electrons are in NH3?
+
NH3 has a total of 8 valence electrons: 5 from nitrogen and 1 from each of the three hydrogen atoms.
Why is the bond angle in NH3 less than 109.5 degrees?
+
The bond angle in NH3 is approximately 107 degrees because the lone pair of electrons repels the bonded pairs more strongly than in a perfect tetrahedral structure.



