NH3O Lewis Structure: A Step-by-Step Guide
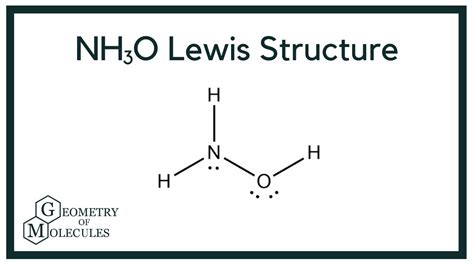
Understanding the NH3O Lewis Structure is crucial for anyone studying chemistry, particularly in the context of chemical bonding and molecular geometry. This step-by-step guide will walk you through the process of drawing the Lewis structure for NH3O, also known as the ammonium hydroxide molecule. Whether you’re a student, educator, or chemistry enthusiast, this guide will provide clear, SEO-optimized insights to enhance your understanding.
What is the NH3O Lewis Structure?

The NH3O Lewis Structure represents the arrangement of atoms and electrons in the ammonium hydroxide molecule. It helps visualize the bonding and lone pairs of electrons, which are essential for predicting molecular properties. By mastering this structure, you’ll gain a deeper understanding of chemical reactions and molecular behavior.
Step 1: Determine the Total Number of Valence Electrons

To begin drawing the NH3O Lewis Structure, first calculate the total number of valence electrons. Here’s how:
- Nitrogen (N): 5 valence electrons
- Hydrogen (H): 1 valence electron (x3) = 3 electrons
- Oxygen (O): 6 valence electrons
Total valence electrons = 5 (N) + 3 (H) + 6 (O) = 14 electrons
📌 Note: Hydrogen atoms always have only one valence electron and form single bonds.
Step 2: Identify the Central Atom
In NH3O, Nitrogen (N) is the central atom because it is less electronegative than oxygen and can form more bonds. Arrange the hydrogen and oxygen atoms around it.
Step 3: Draw Single Bonds Between Atoms

Connect the atoms with single bonds. Nitrogen will be bonded to three hydrogen atoms and one oxygen atom. This uses up 8 electrons (4 bonds).
Step 4: Distribute Remaining Electrons

After forming the bonds, you have 6 remaining electrons. Place these on the oxygen atom as lone pairs to complete its octet.
Step 5: Check for Octet Rule Compliance
Ensure all atoms (except hydrogen) have a complete octet:
- Nitrogen: 4 bonding electrons (satisfies octet)
- Oxygen: 6 electrons (2 lone pairs + 2 bonding electrons)
📌 Note: Hydrogen only needs 2 electrons to be stable.
Final NH3O Lewis Structure
The NH3O Lewis Structure consists of:
- Nitrogen (N) as the central atom
- Three hydrogen (H) atoms bonded to nitrogen
- One oxygen (O) atom bonded to nitrogen with two lone pairs
| Atom | Bonding Electrons | Lone Pairs |
|---|---|---|
| Nitrogen (N) | 4 | 0 |
| Oxygen (O) | 2 | 4 |
| Hydrogen (H) | 2 (each) | 0 |

Checklist for Drawing the NH3O Lewis Structure
- Calculate total valence electrons: 14
- Identify the central atom: Nitrogen (N)
- Draw single bonds: N-H (x3) and N-O
- Distribute remaining electrons: Lone pairs on Oxygen
- Verify octet rule compliance for all atoms
By following these steps, you’ll successfully draw the NH3O Lewis Structure, a fundamental skill in chemistry.
What is the molecular geometry of NH3O?
+The molecular geometry of NH3O is trigonal pyramidal due to the arrangement of atoms and lone pairs around the central nitrogen atom.
Why does NH3O have a tetrahedral electron geometry?
+NH3O has a tetrahedral electron geometry because there are four electron domains around the nitrogen atom: three bonding pairs and one lone pair.
Is NH3O a polar molecule?
+Yes, NH3O is a polar molecule due to the presence of a lone pair on the nitrogen atom and the electronegativity difference between nitrogen, oxygen, and hydrogen.
In summary, mastering the NH3O Lewis Structure involves calculating valence electrons, identifying the central atom, drawing bonds, distributing lone pairs, and verifying octet compliance. This guide ensures you grasp the fundamentals, making it easier to tackle more complex molecules. Whether for academic purposes or professional research, understanding this structure is invaluable in chemistry,lewis structure,molecular geometry,chemical bonding,valence electrons.


