Nickel 2+ Electron Configuration: Simplified Guide
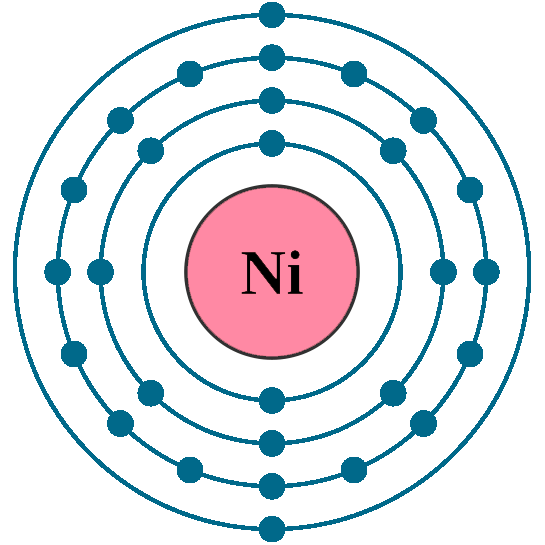
Understanding the Nickel 2+ Electron Configuration is essential for students, chemists, and researchers studying transition metals. Nickel (Ni) is a fascinating element with unique properties, especially in its Ni 2+ form. This guide simplifies the electron configuration of Nickel 2+, making it accessible for both informational and commercial purposes. Whether you’re preparing for an exam or exploring applications in industries like catalysis or electroplating, this post has you covered.
What is Nickel 2+ Electron Configuration?

Nickel (Ni) has an atomic number of 28, and its ground state electron configuration is [Ar] 4s² 3d⁸. When Nickel loses two electrons to form Ni 2+, its electron configuration changes. The Nickel 2+ electron configuration is [Ar] 3d⁸, as the electrons are removed from the 4s orbital first, following the Aufbau principle.
💡 Note: The 4s orbital loses electrons before the 3d orbital because it has higher energy in transition metals.
Why is Nickel 2+ Electron Configuration Important?
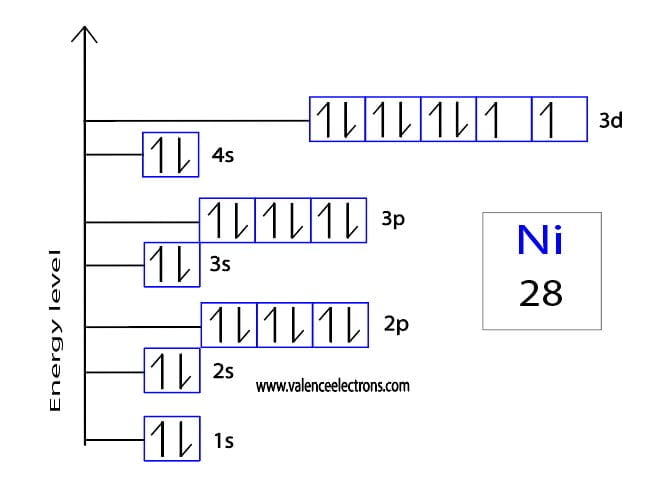
The Ni 2+ electron configuration is crucial for understanding its chemical behavior, magnetic properties, and industrial applications. For instance, Nickel 2+ is widely used in batteries, alloys, and as a catalyst in chemical reactions. Its 3d⁸ configuration contributes to its stability and reactivity, making it a key player in various processes.
Key Applications of Nickel 2+
- Catalysis: Ni 2+ is used in hydrogenation reactions.
- Electroplating: It provides a protective coating for metals.
- Batteries: Nickel-based batteries are popular for their high energy density.
How to Determine Nickel 2+ Electron Configuration

To determine the Nickel 2+ electron configuration, follow these steps:
1. Start with Nickel’s ground state configuration: [Ar] 4s² 3d⁸.
2. Remove two electrons from the highest energy orbital, which is 4s.
3. The resulting configuration is [Ar] 3d⁸.
| Step | Configuration |
|---|---|
| Ground State (Ni) | \[Ar\] 4s² 3d⁸ |
| Remove 2 electrons | \[Ar\] 3d⁸ (Ni 2+) |

✨ Note: Always remove electrons from the outermost orbital first unless specified otherwise.
Checklist for Mastering Nickel 2+ Electron Configuration

- Understand the ground state configuration of Nickel: [Ar] 4s² 3d⁸.
- Know the order of electron removal: 4s before 3d.
- Recognize the final configuration of Ni 2+: [Ar] 3d⁸.
- Explore real-world applications of Nickel 2+ in catalysis, electroplating, and batteries.
In summary, the Nickel 2+ electron configuration is [Ar] 3d⁸, derived by removing two electrons from the 4s orbital. This knowledge is fundamental for understanding its chemical properties and applications in industries. Whether you’re studying for an exam or exploring commercial uses, mastering this concept is a valuable step in your journey.
What is the electron configuration of Nickel 2+?
+The electron configuration of Nickel 2+ (Ni 2+) is \[Ar\] 3d⁸.
Why does Nickel lose electrons from the 4s orbital first?
+In transition metals, the 4s orbital has higher energy than the 3d orbital, so electrons are removed from 4s first.
What are the main applications of Nickel 2+?
+Nickel 2+ is used in catalysis, electroplating, and as a component in batteries.
Nickel 2+ electron configuration,Ni 2+ electron configuration,transition metals,electron configuration,catalysis,electroplating,batteries.



