Sci4 Lewis Structure: Quick Guide & Tips
Understanding the Sci4 Lewis Structure is crucial for students and professionals in chemistry. This guide provides a quick and comprehensive overview, ensuring you grasp the concept efficiently. Whether you're preparing for an exam or working on a project, mastering the Lewis structure of Sci4 will enhance your chemical knowledge. Let’s dive into the essentials, tips, and best practices to make this process seamless.
What is Sci4 Lewis Structure?
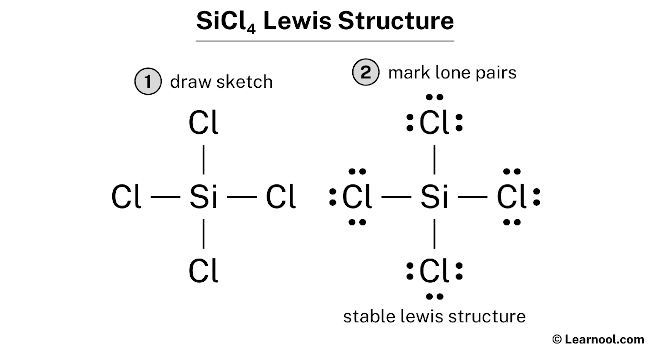
The Sci4 Lewis Structure represents the arrangement of atoms, bonds, and lone pairs in a molecule. It’s a fundamental concept in chemistry, helping visualize the electron distribution. For Sci4, also known as silicon tetrahydride, the structure involves silicon (Si) as the central atom, surrounded by four hydrogen (H) atoms. Understanding this structure is key to predicting molecular geometry and properties.
📌 Note: Silicon tetrahydride (Sci4) is a simple covalent molecule, making its Lewis structure straightforward to draw.
Steps to Draw Sci4 Lewis Structure

Follow these step-by-step instructions to accurately draw the Sci4 Lewis Structure:
- Step 1: Count Total Valence Electrons – Silicon has 4 valence electrons, and each hydrogen has 1. Total: 4 (Si) + 4*1 (H) = 8 electrons.
- Step 2: Place Atoms – Position silicon in the center and hydrogen atoms around it.
- Step 3: Form Bonds – Connect each hydrogen to silicon with a single bond, using 4 electrons (2 bonds).
- Step 4: Complete Octets – Ensure all atoms satisfy the octet rule. Hydrogen needs 2 electrons, which is fulfilled by the single bond.
- Step 5: Verify Stability – Check if the structure is stable with no leftover electrons.
Tips for Mastering Sci4 Lewis Structure

Here are some valuable tips to simplify the process:
- Practice Regularly – Repetition helps solidify your understanding.
- Use Visual Aids – Diagrams and charts can make complex structures easier to grasp.
- Understand Electronegativity – Knowing how atoms share electrons aids in predicting bond formation.
- Consult Resources – Refer to textbooks or online tools for additional guidance.
Common Mistakes to Avoid
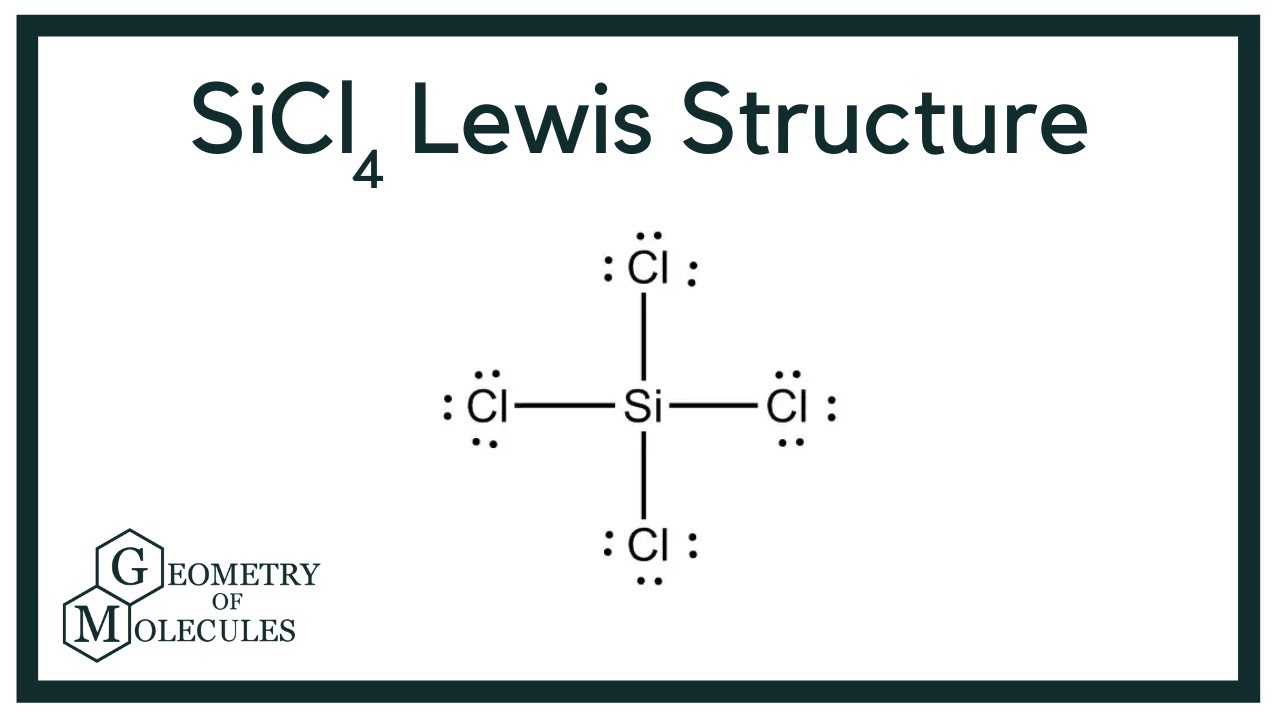
Steer clear of these common errors when drawing the Sci4 Lewis Structure:
- Incorrect Electron Count – Double-check the total valence electrons to avoid mistakes.
- Overlooking Octet Rule – Ensure all atoms (except hydrogen) have 8 electrons in their outer shell.
- Misplacing Atoms – Always place the least electronegative atom (silicon) in the center.
Checklist: Drawing Sci4 Lewis Structure
- Count total valence electrons.
- Place silicon as the central atom.
- Connect hydrogen atoms with single bonds.
- Verify octet rule compliance.
- Check structure stability.
Mastering the Sci4 Lewis Structure is essential for anyone studying chemistry. By following the steps, tips, and avoiding common mistakes, you’ll be able to draw the structure confidently. Practice regularly and use resources to enhance your skills. Remember, understanding molecular structures like Sci4 is a stepping stone to advanced chemistry concepts. Lewis structure,molecular geometry,chemical bonding.
What is the molecular geometry of Sci4?
+Sci4 has a tetrahedral geometry, with silicon at the center and hydrogen atoms at the vertices.
How many bonds does silicon form in Sci4?
+Silicon forms four single bonds with hydrogen atoms in Sci4.
Why is the Sci4 Lewis Structure important?
+It helps predict the molecule’s geometry, reactivity, and physical properties, essential for chemical analysis.



