Understanding SCN Formal Charge: A Quick Guide

Understanding SCN Formal Charge: A Quick Guide
If you're studying chemistry, you've likely encountered the concept of formal charge, particularly when dealing with polyatomic ions like SCN- (thiocyanate). Calculating the formal charge is essential for understanding the distribution of electrons and the stability of molecules. In this guide, we'll break down the process of determining the SCN formal charge, providing clear steps and insights to help you master this fundamental concept.
What is Formal Charge?

Formal charge is a tool used to determine the charge distribution within a molecule or ion. It helps identify which atoms carry a positive, negative, or neutral charge. For the SCN- ion, understanding the formal charge on each atom (Sulfur, Carbon, and Nitrogen) is crucial for predicting its reactivity and stability. (formal charge calculation, SCN ion structure)
How to Calculate SCN Formal Charge
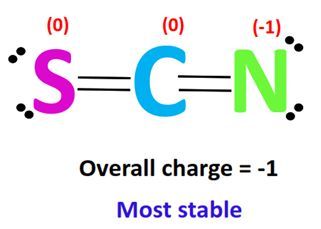
Calculating the formal charge involves a simple formula: Formal Charge = Valence Electrons – (Lone Pair Electrons + Bonding Electrons / 2). Let’s apply this to the SCN- ion step by step.
Step 1: Determine Valence Electrons
- Sulfur (S): 6 valence electrons
- Carbon (C): 4 valence electrons
- Nitrogen (N): 5 valence electrons
Step 2: Identify Bonding and Lone Pair Electrons
In SCN-, the structure is S-C≡N. Here’s the electron distribution:
- Sulfur (S): 2 lone pairs, 2 bonding electrons
- Carbon (C): 0 lone pairs, 4 bonding electrons
- Nitrogen (N): 2 lone pairs, 2 bonding electrons
Step 3: Apply the Formal Charge Formula
| Atom | Valence Electrons | Lone Pair Electrons | Bonding Electrons | Formal Charge |
|---|---|---|---|---|
| S | 6 | 4 | 2 | 6 - (4 + 2/2) = 0 |
| C | 4 | 0 | 4 | 4 - (0 + 4/2) = 0 |
| N | 5 | 4 | 2 | 5 - (4 + 2/2) = 0 |
📌 Note: The overall charge of SCN- is -1, but individual formal charges help in understanding electron distribution.
Why SCN Formal Charge Matters

Understanding the formal charge of SCN- is vital for predicting its behavior in chemical reactions. It helps in:
- Determining the most stable resonance structure.
- Identifying reactive sites in the molecule.
- Explaining the ion's role in coordination compounds.
By mastering this concept, you'll gain deeper insights into chemical bonding and molecular interactions. (chemical bonding, molecular stability)
Checklist for Calculating Formal Charge

- Identify the valence electrons of each atom.
- Determine the number of lone pair and bonding electrons.
- Apply the formal charge formula for each atom.
- Verify the overall charge of the ion matches the given value.
With this quick guide, you're now equipped to tackle SCN formal charge calculations confidently. Practice with other molecules to solidify your understanding. (formal charge practice, chemistry tutorials)
What is the formal charge of SCN-?
+The overall formal charge of SCN- is -1. Individual formal charges for S, C, and N are typically 0 in the most stable resonance structure.
Why is formal charge important in chemistry?
+Formal charge helps predict molecular stability, reactivity, and the distribution of electrons in a molecule or ion.
How do I calculate formal charge for other molecules?
+Use the formula: Formal Charge = Valence Electrons – (Lone Pair Electrons + Bonding Electrons / 2). Follow the steps outlined in this guide for any molecule.
By following this guide, you’ll be able to calculate formal charges efficiently, enhancing your understanding of chemical structures. Keep practicing, and soon you’ll tackle complex molecules with ease! (chemistry learning, molecular structure)



