Optimal Carbonyl Stretching Frequency Explained Mastering Carbonyl IR Stretching Frequencies Key Insights on Carbonyl Stretching Range Carbonyl Stretching: Frequency and Analysis Understanding Carbonyl Vibrational Frequencies
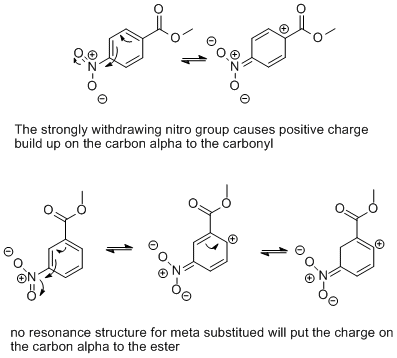
Understanding the optimal carbonyl stretching frequency is crucial for chemists, researchers, and students analyzing organic compounds using infrared (IR) spectroscopy. Carbonyl groups (C=O) are prevalent in various molecules, and their vibrational frequencies provide valuable insights into molecular structure and bonding. This post delves into carbonyl IR stretching frequencies, their analysis, and practical tips for mastering this essential concept in spectroscopy.
Mastering Carbonyl IR Stretching Frequencies

Carbonyl stretching frequencies typically range between 1600–1800 cm-1 in IR spectroscopy. However, the exact frequency depends on factors like electronegativity, molecular environment, and hydrogen bonding. For instance, a ketone may show a sharper peak around 1715 cm-1, while a carboxylic acid exhibits a broader peak around 1700–1725 cm-1 due to hydrogen bonding. (Carbonyl Stretching: Frequency and Analysis, IR Spectroscopy Basics, Molecular Vibrations)
Key Insights on Carbonyl Stretching Range
- Electronegativity: Higher electronegativity shifts the frequency to higher wavenumbers.
- Conjugation: Conjugated carbonyls show lower frequencies (e.g., 1680–1690 cm-1).
- Hydrogen Bonding: Broadens peaks and slightly lowers frequency.
Understanding Carbonyl Vibrational Frequencies

The carbonyl stretch is a result of the C=O bond vibration, which is highly sensitive to its chemical environment. Analyzing this frequency helps identify functional groups and confirm molecular structures. For example, aldehydes typically stretch around 1720–1740 cm-1, while esters appear at 1730–1750 cm-1. (Optimal Carbonyl Stretching Frequency Explained, Carbonyl Stretching Range)
| Functional Group | Typical Frequency (cm-1) |
|---|---|
| Ketone | 1710–1720 |
| Aldehyde | 1720–1740 |
| Carboxylic Acid | 1700–1725 |
| Ester | 1730–1750 |

📌 Note: Always consider the broader molecular context when interpreting carbonyl stretches, as overlapping peaks can occur.
Checklist for Analyzing Carbonyl Stretching Frequencies
- Identify the functional group based on the frequency range.
- Check for peak broadening indicating hydrogen bonding.
- Compare with known standards for accurate identification.
- Consider conjugation and electronegativity effects.
In summary, mastering carbonyl IR stretching frequencies requires understanding how molecular environments influence vibrational frequencies. By focusing on key factors like electronegativity, conjugation, and hydrogen bonding, you can accurately analyze and interpret spectroscopic data. (Carbonyl Stretching: Frequency and Analysis, Key Insights on Carbonyl Stretching Range)
What is the typical range for carbonyl stretching frequencies?
+
Carbonyl stretching frequencies typically range between 1600–1800 cm-1 in IR spectroscopy.
How does hydrogen bonding affect carbonyl stretches?
+
Hydrogen bonding broadens the carbonyl peak and slightly lowers its frequency.
Why do conjugated carbonyls show lower frequencies?
+
Conjugation delocalizes electrons, reducing the bond strength and lowering the vibrational frequency.



