Tertiary Amine FTIR: Key Spectral Insights Explained

Fourier-Transform Infrared Spectroscopy (FTIR) is a powerful analytical technique used to identify and characterize organic compounds, including tertiary amines. By analyzing the vibrational modes of molecules, FTIR provides valuable insights into the structural features of these compounds. This blog post delves into the key spectral insights of tertiary amine FTIR, offering both informative and commercial perspectives for readers. Whether you’re a researcher, chemist, or industry professional, understanding these spectral characteristics is crucial for accurate compound identification and application. (FTIR spectroscopy, tertiary amine analysis, organic compound characterization)
Understanding Tertiary Amines and FTIR Basics

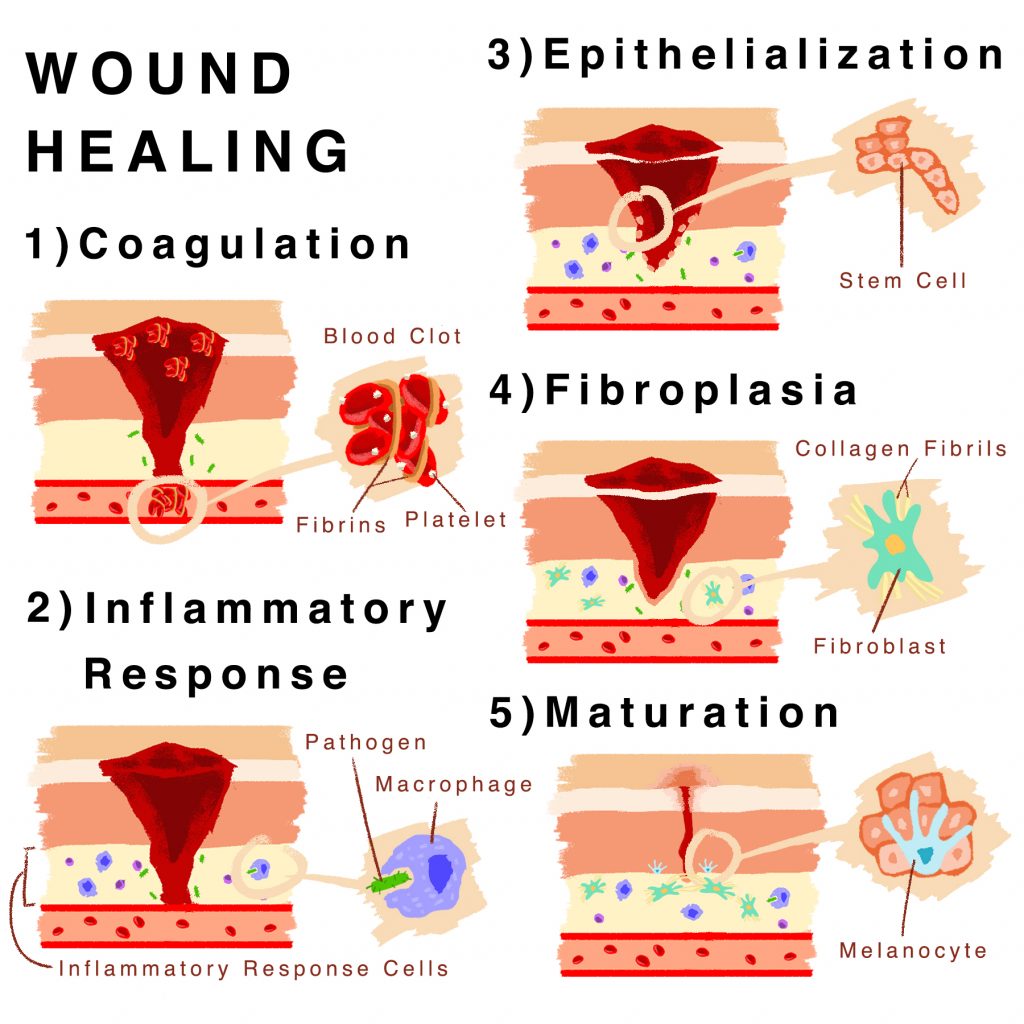
Tertiary amines are nitrogen-containing compounds where the nitrogen atom is bonded to three organic groups. Their unique structure results in distinct FTIR spectral features, making them identifiable through this technique. FTIR works by measuring the absorption of infrared light by a sample, producing a spectrum that reflects the molecule’s vibrational frequencies.
Key functional groups in tertiary amines, such as the C-N stretch and N-H bend, appear at specific wavenumber ranges, typically between 1000–1300 cm⁻¹ and 1500–1600 cm⁻¹, respectively. Understanding these peaks is essential for spectral interpretation. (FTIR basics, tertiary amine structure, C-N stretch, N-H bend)
Key Spectral Insights for Tertiary Amine FTIR
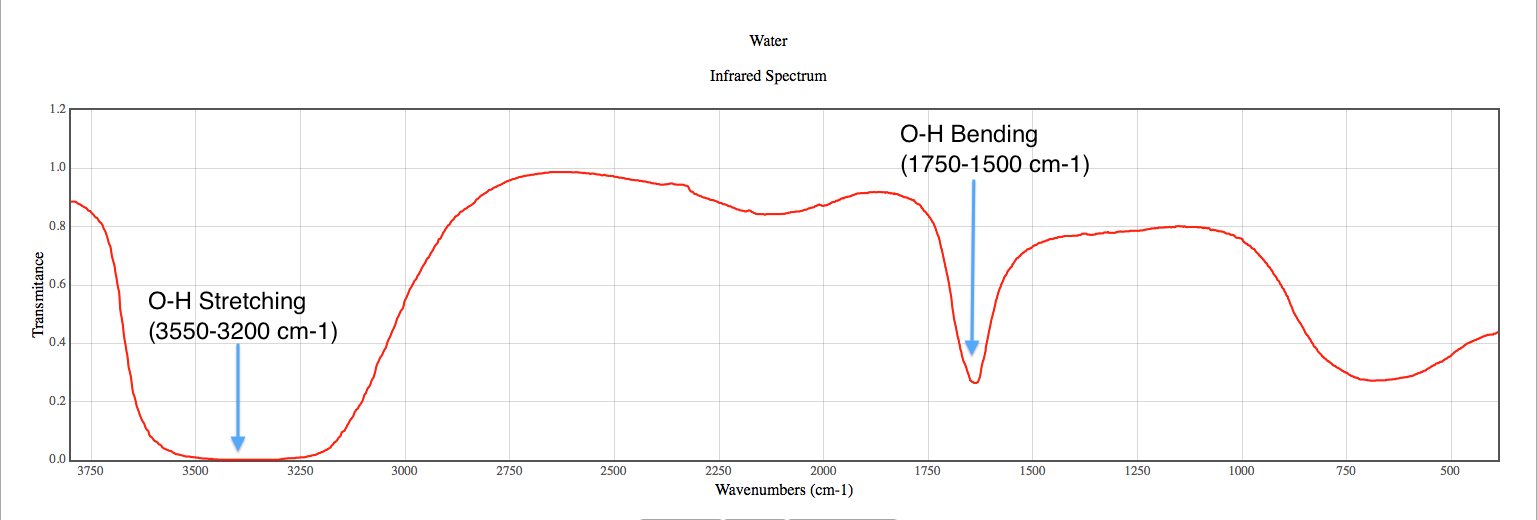
1. C-N Stretching Vibrations
The C-N stretch is a hallmark of tertiary amines in FTIR spectra. This peak typically appears in the 1000–1300 cm⁻¹ region. The exact position depends on the substituents attached to the nitrogen atom. For example, alkyl-substituted tertiary amines show sharper peaks compared to aromatic-substituted ones.
📌 Note: The absence of a C-N stretch peak may indicate the compound is not a tertiary amine.
2. N-H Bending Vibrations
While tertiary amines lack a hydrogen directly bonded to nitrogen, secondary amines or impurities may show N-H bending peaks around 1500–1600 cm⁻¹. Identifying these peaks helps distinguish between secondary and tertiary amines.
3. Aromatic and Aliphatic Contributions
If the tertiary amine contains aromatic rings or aliphatic chains, additional peaks will appear. Aromatic C-H stretches are observed around 3000–3100 cm⁻¹, while aliphatic C-H stretches appear at 2800–3000 cm⁻¹.
| Peak Region (cm⁻¹) | Functional Group | Significance |
|---|---|---|
| 1000–1300 | C-N Stretch | Confirms tertiary amine presence |
| 1500–1600 | N-H Bend | Indicates secondary amine impurities |
| 3000–3100 | Aromatic C-H Stretch | Identifies aromatic substituents |

Applications of Tertiary Amine FTIR Analysis

For commercial-intent visitors, tertiary amine FTIR analysis is vital in industries like pharmaceuticals, polymers, and catalysis. It ensures product quality, identifies contaminants, and aids in process optimization.
- Pharmaceuticals: Verify the purity of active ingredients.
- Polymers: Monitor amine-based curing agents in epoxy resins.
- Catalysis: Characterize amine-based catalysts for chemical reactions.
(Pharmaceutical analysis, polymer characterization, catalysis applications)
Checklist for Tertiary Amine FTIR Analysis
- Identify C-N stretch in the 1000–1300 cm⁻¹ region.
- Check for N-H bend peaks to rule out secondary amines.
- Analyze aromatic/aliphatic peaks for substituent identification.
- Compare with reference spectra for accurate characterization.
What is the primary FTIR peak for tertiary amines?
+The primary peak is the C-N stretch, appearing in the 1000–1300 cm⁻¹ region.
Can FTIR distinguish between secondary and tertiary amines?
+Yes, secondary amines show N-H bending peaks around 1500–1600 cm⁻¹, absent in tertiary amines.
How are aromatic substituents identified in tertiary amines using FTIR?
+Aromatic C-H stretches appear around 3000–3100 cm⁻¹ in the FTIR spectrum.
In summary, tertiary amine FTIR analysis provides critical spectral insights for compound identification and industrial applications. By focusing on key peaks like the C-N stretch and understanding substituent contributions, researchers and professionals can ensure accurate characterization and quality control. Whether for academic research or commercial purposes, mastering these spectral features is indispensable. (FTIR spectroscopy, tertiary amine analysis, organic compound characterization)