Understanding Conjugated Double Bonds: A Simple Guide
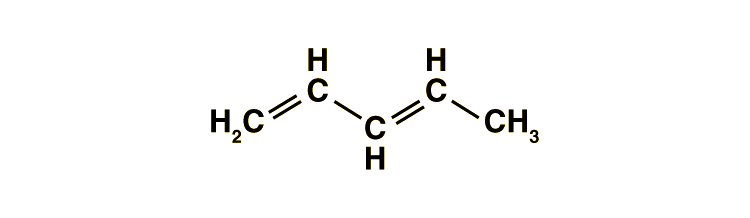
Conjugated double bonds are a fascinating aspect of organic chemistry, playing a crucial role in the properties and reactivity of molecules. These systems, characterized by alternating single and double bonds, are found in various natural and synthetic compounds, from pigments to pharmaceuticals. Understanding conjugated double bonds is essential for students, researchers, and professionals in chemistry, biology, and materials science. In this guide, we’ll break down the concept into simple terms, explore their significance, and provide practical insights for both informational and commercial purposes. (conjugated double bonds, organic chemistry, molecular structure)
What Are Conjugated Double Bonds?

Conjugated double bonds refer to a system where double bonds (C=C) alternate with single bonds (C-C) in a molecule. This arrangement creates a delocalized electron system, which gives conjugated molecules unique properties. For example, they often exhibit enhanced stability, distinct colors, and specific reactivity patterns. Common examples include beta-carotene (responsible for the orange color of carrots) and benzene, a fundamental aromatic compound. (conjugated systems, delocalized electrons, aromatic compounds)
Why Are Conjugated Double Bonds Important?

Conjugated double bonds are vital in both natural and synthetic applications. They influence:
- Color and Light Absorption: Many pigments and dyes contain conjugated systems, absorbing specific wavelengths of light to produce color. (pigments, light absorption)
- Chemical Reactivity: Conjugation affects how molecules react, making them useful in organic synthesis and drug development. (chemical reactivity, organic synthesis)
- Material Properties: Conjugated polymers are used in electronics, such as OLEDs and solar cells, due to their conductivity. (conjugated polymers, electronics)
How to Identify Conjugated Double Bonds

Identifying conjugated systems is straightforward if you know what to look for:
- Alternating Bonds: Check for alternating double and single bonds in a carbon chain. (alternating bonds)
- Delocalized Electrons: Conjugated systems often have electrons that are not confined to a single bond. (delocalized electrons)
- Aromaticity: Benzene and its derivatives are classic examples of conjugated aromatic systems. (aromaticity)
📌 Note: Not all alternating double bonds are conjugated. Ensure there are no interrupting groups like alkyl chains. (conjugated systems, alkyl chains)
Applications of Conjugated Double Bonds
Conjugated systems have diverse applications across industries:
| Field | Application |
|---|---|
| Biochemistry | Photosynthesis (chlorophyll) |
| Materials Science | Conductive polymers in electronics |
| Pharmaceuticals | Drug design and synthesis |

For commercial purposes, companies leverage conjugated systems to develop innovative products like organic LEDs and advanced pharmaceuticals. (commercial applications, conductive polymers, drug design)
Summary and Checklist

To recap, conjugated double bonds are essential in chemistry due to their unique properties and applications. Here’s a quick checklist to remember:
- Identify alternating double and single bonds.
- Understand delocalized electron systems.
- Explore applications in pigments, electronics, and pharmaceuticals.
Whether you’re a student or a professional, mastering conjugated systems opens doors to exciting possibilities. (conjugated systems, chemistry applications)
What makes conjugated double bonds special?
+Conjugated double bonds have delocalized electrons, which give them unique properties like stability, color, and reactivity. (delocalized electrons, unique properties)
Where are conjugated systems found in nature?
+They are found in pigments like chlorophyll and beta-carotene, as well as in aromatic compounds like benzene. (natural pigments, aromatic compounds)
How are conjugated polymers used in technology?
+Conjugated polymers are used in electronics for their conductivity, such as in OLEDs and solar cells. (conjugated polymers, electronics)
Conjugated double bonds are a cornerstone of organic chemistry, bridging the gap between theoretical concepts and real-world applications. By understanding their structure and properties, you can appreciate their role in everything from natural pigments to cutting-edge technology. Whether you’re exploring this topic for academic or commercial purposes, the knowledge gained here will undoubtedly prove valuable. (organic chemistry, real-world applications)



