Benzene IR Spectra: Key Peaks Explained Understanding Benzene IR Spectroscopy Basics Decoding Benzene’s IR Spectrum Features Essential Guide to Benzene IR Spectra Benzene IR Spectra: Quick Analysis Tips

Infrared (IR) spectroscopy is a powerful tool for analyzing organic compounds, and benzene, with its unique aromatic structure, presents distinct spectral features. Understanding benzene IR spectra is essential for chemists, researchers, and students alike. This guide breaks down the key peaks, their origins, and quick analysis tips to help you decode benzene’s IR spectrum with confidence. Whether you're a beginner or an expert, this post will enhance your knowledge of benzene IR spectroscopy basics, making complex data easier to interpret.
Understanding Benzene IR Spectroscopy Basics

Benzene (C6H6) is an aromatic hydrocarbon with a symmetrical ring structure. Its IR spectrum reflects the vibrations of its C-H and C-C bonds. Key regions to focus on include the functional group region (4000–1300 cm-1) and the fingerprint region (1300–650 cm-1). Below are the fundamental principles to grasp:
- C-H Stretching: Aromatic C-H bonds typically appear between 3000–3100 cm-1.
- C-C Stretching: Aromatic C-C vibrations are found in the 1600–1400 cm-1 range.
- Fingerprint Region: Unique patterns here help confirm benzene’s presence.
📌 Note: Benzene’s spectrum lacks aliphatic C-H peaks, distinguishing it from non-aromatic compounds.
Decoding Benzene’s IR Spectrum Features

Benzene’s IR spectrum is characterized by specific peaks that correspond to its molecular vibrations. Here’s a breakdown of the most important features:
| Peak (cm-1) | Vibration Type | Significance |
|---|---|---|
| 3030 | Aromatic C-H Stretch | Indicates the presence of aromatic rings. |
| 1600–1450 | C-C Stretching | Confirms aromaticity and ring structure. |
| ~700 | Out-of-Plane C-H Bend | Unique to monosubstituted benzenes. |
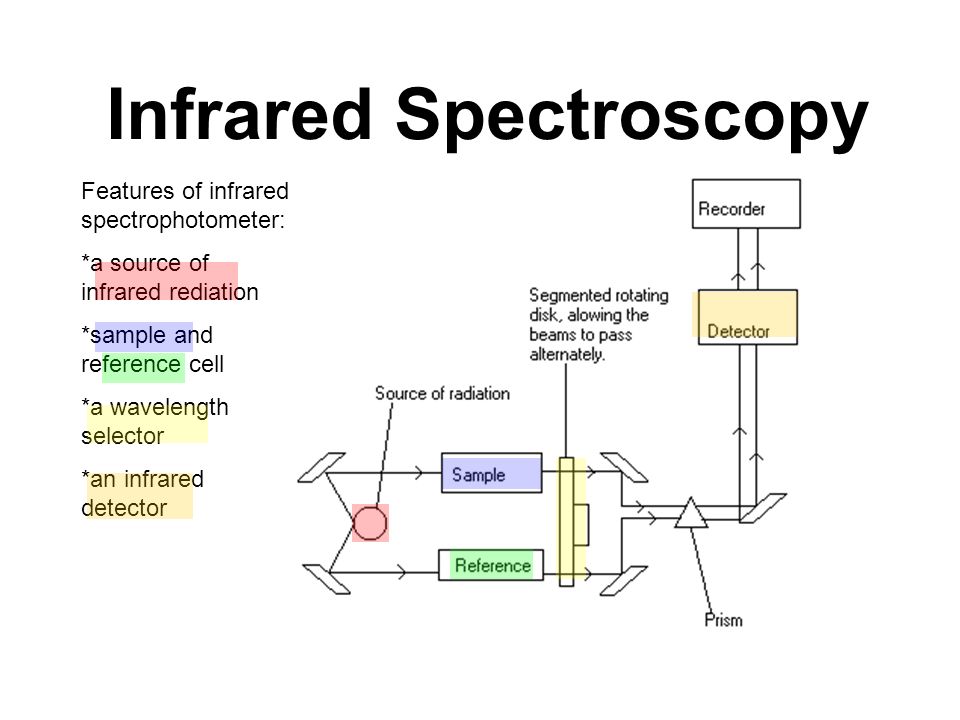
These peaks are critical for identifying benzene in mixtures or confirming its purity. For substituted benzenes, additional peaks may appear, depending on the substituents.
Essential Guide to Benzene IR Spectra

To master benzene IR spectra analysis, follow these steps:
- Identify Aromatic C-H Peaks: Look for sharp absorptions around 3030 cm-1.
- Analyze C-C Vibrations: Check the 1600–1400 cm-1 region for aromatic ring confirmation.
- Examine the Fingerprint Region: Compare patterns with known benzene spectra.
- Consider Substituents: Note additional peaks if benzene is substituted.
✨ Note: Always compare your spectrum with a reference library for accuracy.
Benzene IR Spectra: Quick Analysis Tips

For efficient analysis, keep these tips in mind:
- Focus on Key Peaks: Prioritize aromatic C-H and C-C stretches.
- Use Reference Spectra: Compare with known benzene spectra for validation.
- Check for Purity: Ensure no additional peaks indicate impurities.
- Note Substituent Effects: Substituents can shift or add peaks.
By following these tips, you’ll streamline your analysis and improve accuracy. (Benzene IR Spectra Analysis, IR Spectroscopy Tips, Aromatic Compound Identification)
Summary Checklist for Benzene IR Spectra Analysis

- ✅ Identify aromatic C-H peaks at ~3030 cm-1.
- ✅ Confirm C-C stretching in the 1600–1400 cm-1 range.
- ✅ Analyze the fingerprint region for unique patterns.
- ✅ Compare with reference spectra for accuracy.
- ✅ Account for substituent effects in substituted benzenes.
Mastering benzene IR spectra is a valuable skill for anyone working with aromatic compounds. By understanding key peaks, following analysis tips, and using reference spectra, you can confidently interpret benzene’s IR spectrum. Whether you're in research, education, or industry, this knowledge will enhance your spectroscopic expertise. (IR Spectroscopy Basics, Benzene Spectrum Features, Essential Guide to IR Analysis)
What are the key peaks in benzene’s IR spectrum?
+
The key peaks include aromatic C-H stretching at ~3030 cm-1, C-C stretching between 1600–1400 cm-1, and out-of-plane C-H bending around 700 cm-1.
How does benzene’s IR spectrum differ from aliphatic compounds?
+
Benzene lacks aliphatic C-H peaks (2800–3000 cm-1) and shows distinct aromatic C-H and C-C vibrations.
Can substituents affect benzene’s IR spectrum?
+
Yes, substituents can shift peaks or introduce new ones, depending on their nature and position.